Sequence information
DRAVP ID DRAVPc002
Name Boceprevir
Sequence Not available
Molecular Formula C27H45N5O5
Condition/Disease Chronic HCV infection
Group Approved, Withdrawn
Type Peptidomimetic
Description Boceprevir is a hepatitis C virus NS3/4A protease inhibitor used in combination with other medications to treat chronic hepatitis C genotype 1 infection. Initially approved for use in 2012, it was withdrawn in 2015 because of the availability of more effective and better tolerated all oral regimens of direct acting antiviral agents. Boceprevir is available as a fixed dose product (tradename Victrelis) used for the treatment of chronic Hepatitis C. Approved in May 2011 by the FDA, Victrelis is indicated for the treatment of HCV genotype 1 in combination with Ribavirin, Peginterferon alfa-2a, and Peginterferon alfa-2b.
Active sequence/Structure
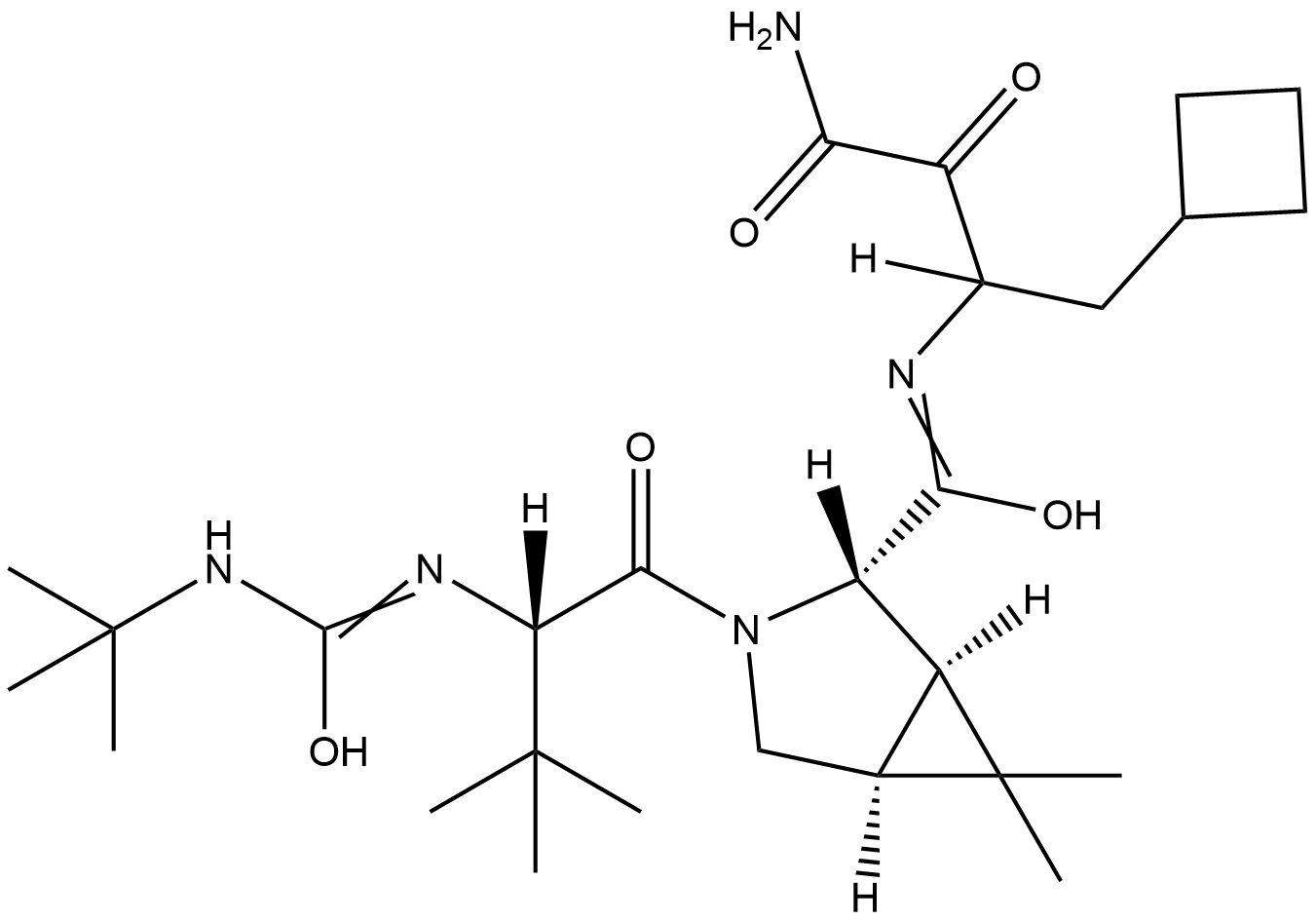
Comment
No comments found.
External Links
DrugBank Accession Number DB08873
Pubchem ID 10324367
CHEMBL ID CHEMBL218394
UNII 89BT58KELH
CAS 394730-60-0
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT01335529 | Boceprevir in HIV-HCV Coinfected Patients Who Have Failed to a Previous Therapy With Peg-Interferon/Ribavirin (BocepreVIH) | HCV Coinfection / Human Immunodeficiency Virus Type 1 (HIV-1) Infection | Completed | Phase 2 | ANRS, Emerging Infectious Diseases |
| NCT01457937 | Boceprevir/PegIFN α-2b/Riba in HCV+ Gt1 Menopausal Women, Nonresponders to PegIFN/Riba or Treatment-naives (MEN_BOC) (MEN_BOC) | Chronic Hepatitis C Virus (HCV) Infection / Menopause | Unknown | Phase 3 | University of Modena and Reggio Emilia |
| NCT01731301 | A Pilot Study to Treat Patients With Chronic Hepatitis C Virus (HCV) Genotype 1 and End-Stage Renal Disease (ESRD) | Chronic Hepatitis C Virus (HCV) Infection / End Stage Renal Diseases | Unknown | Phase 4 | Liver Institute of Virginia |
| NCT01403181 | Effect of Boceprevir on HCV-specific T Cell Responses (Boce-Par) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Not available | Azienda Ospedaliero-Universitaria di Parma |
| NCT01663922 | Boceprevir and Ucalm (St John&Apos;s Wort) | Hepatitis C Virus (HCV) Infection | Completed | Phase 1 | St Stephens Aids Trust |
| NCT01653236 | Boceprevir With Peginterferon Alfa-2b and Ribavirin in the Treatment-naive Patients Infected With Genotype 4 Chronic Hepatitis C Infection | Genotype 4 Chronic Hepatitis C Infection | Unknown | Phase 3 | Theodor Bilharz Research Institute |
| NCT01465516 | Treating Hispanic Patients Diagnosed With Hepatitis C Using Boceprevir | Hepatitis C Virus (HCV) Infection | Terminated (The study was terminated and recruitment was capped because of the new changes in hepati | Not available | Arrowhead Regional Medical Center |
| NCT01718301 | HIV Patients With Chronic Hepatitis C Genotype 1 Infection Who Failed Previously to Peginterferon /Ribavirin (BOC-HIV) | Co-Infection / Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | Anna Cruceta |
| NCT01443923 | Boceprevir Drug Combination for Hepatitis C Treatment in People With and Without HIV | Hepatitis / Human Immunodeficiency Virus Infection(HIV)/Acquired Immunodeficiency Syndrome (AIDS) | Terminated (Unable to complete enrollment due to newly approved treatment options.) | Phase 4 | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT01641666 | Safety and Tolerability of Boceprevir in Combination With Peginterferon Alfa-2b Plus Ribavirin for the Treatment of Vietnamese Subjects With Chronic Hepatitis C Genotype 1 (P08599) | Chronic Hepatitis C Virus (HCV) Infection | Withdrawn | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01585584 | Triple-Therapy in Patients With HCV Genotype 3 Who Previously Failed Treatment (LeeG3) | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | University of Calgary |
| NCT02112630 | Boceprevir in End Stage Renal Disease (ESRD) | Hepatitis C Virus (HCV) Infection/End Stage Renal Disease | Withdrawn (Poor enrollment) | Not Applicable | Columbia University |
| NCT02204475 | Grazoprevir (MK-5172)/Elbasvir (MK-8742) Versus Boceprevir/Pegylated Interferon/Ribavarin for Chronic Hepatitis C Infection (MK-5172-066) | Hepatitis C Virus (HCV) Infection | Withdrawn | Phase 3 | Merck Sharp & Dohme LLC |
| NCT00160251 | Boceprevir (SCH 503034) Plus Peg-Intron, With and Without Added Ribavirin, in Patients With Chronic Hepatitis C, Genotype 1, Who Did Not Respond to Previous Treatment With Peginterferon Alfa Plus Riba | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Merck Sharp & Dohme LLC |
| NCT01912495 | Dutch Acute HCV in HIV Study (DAHHS) (DAHHS) | Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 2 | Erasmus Medical Center |
| NCT01482403 | A Study of Mericitabine in Combination With Boceprevir and Pegasys/Copegus in Patients With Chronic Hepatitis CA Study of Mericitabine in Combination With Boceprevir and Pegasys/Copegus in Patients Wi | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Hoffmann-La Roche |
| NCT01591460 | A Triple Combination Therapy Study of Boceprevir, Pegasys and Copegus in Previously Untreated Patients With Genotype 1 Chronic Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 4 | Hoffmann-La Roche |
| NCT02118597 | An Observational Study Examining the Use of Triple Combination Therapy With Boceprevir, Peginterferon Alfa-2a and Ribavirin in the Re-Treatment of Chronic Hepatitis C Patients | Chronic Hepatitis C Virus (HCV) Infection | Terminated (Study terminated due to the Sponsor's decision.) | Not available | Hoffmann-La Roche |
| NCT01446250 | Alisporivir (Deb025) and Boceprevir Triple Therapies in African American Participants Not Previously Treated for Chronic Hepatitis C Genotype 1 | Hepatitis C Virus (HCV) Infection | Terminated | Phase 3 | Debiopharm International SA |
| NCT01463956 | Efficacy of PegInterferon-Ribavirin-Boceprevir Therapy in Patients Infected With G1 HCV With Cirrhosis, Awaiting Liver Transplantation | Hepatitis C Virus (HCV) Infection / Liver Cirrhosis, Experimental | Completed | Phase 2 | French National Agency for Research on AIDS and Viral Hepatitis |
| NCT02113631 | Comparative Effectiveness and Tolerability of Boceprevir vs Telaprevir | Chronic Hepatitis C Virus (HCV) Infection /Cirrhosis | Completed | Not applicable | Louis Stokes VA Medical Center |
| NCT01925183 | Individualized Triple-therapy Using Boceprevir in HIV-positive Patients With Hepatitis C (HIVCOBOC-RGT) | Chronic Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Markus Peck-Radosavljevic |
| NCT01770223 | A Study of Viral Response to Triple Therapy in Hepatitis C Virus-Infected Participants With Insulin Resistance Who Failed Dual Therapy (MK-3034-113) | Hepatitis C Virus (HCV) Infection | Withdrawn | Phase 4 | Merck Sharp & Dohme LLC |
| NCT01447446 | An Observational Study on Dual And Triple Therapies Based on Peginterferon Alfa (e.g. Pegasys) in Patients With Chronic Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection | Completed | Not available | Hoffmann-La Roche |
| NCT00423670 | Safety and Efficacy of SCH 503034 in Previously Untreated Subjects With Chronic Hepatitis C Infected With Genotype 1 (Study P03523) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Merck Sharp & Dohme LLC |
| NCT01396005 | A Study to Evaluate the Pharmacokinetic Effect of SCH 503034 (Boceprevir) on Methadone or Buprenorphine/Naloxone Plasma Concentrations (P08123) | Hepatitis C Virus (HCV) Infection | Completed | Phase 1 | Merck Sharp & Dohme LLC |
| NCT01181804 | Comparison of Safety and Resulting Blood Level Profiles After Administration of a New Boceprevir Tablet Versus Its Current Capsule Formulation for Treatment of Chronic Hepatitis C (P06992)(COMPLETED) | Hepatitis C Virus (HCV) Infection | Completed | Phase 1 | Merck Sharp & Dohme LLC |
| NCT00959699 | A Phase 2b, Safety and Efficacy Study of Boceprevir in Patients Coinfected With HIV and Hepatitis C (P05411 AM4) | Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 2 | Merck Sharp & Dohme LLC |
| NCT00845065 | Boceprevir in Combination With Peginterferon Alfa-2a and Ribavirin in Participants With Chronic Hepatitis C Genotype 1 Who Failed Prior Treatment With Peginterferon/Ribavirin (Study P05685AM2)(COMPLET | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT00708500 | Boceprevir in Subjects With Chronic Hepatitis C Genotype 1 Who Failed Prior Treatment With Peginterferon/Ribavirin (Study P05101AM3)(COMPLETED) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT02758509 | Impact of Antiviral Therapy on Gastroesophageal Varices. | Chronic Hepatitis C Virus (HCV) Infection | Completed | Not available | Parc de Salut Mar |
| NCT00705432 | Safety and Efficacy of Boceprevir in Previously Untreated Subjects With Chronic Hepatitis C Genotype 1 (Study P05216AM2) (COMPLETED) (SPRINT-2) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01945294 | Short Duration Versus Standard Response-Guided Therapy With Boceprevir Combined With PegIntron and Ribavirin in Previously Untreated Non-Cirrhotic Asian Participants With Chronic HCV Genotype 1 (MK-30 | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01756079 | A Study to Evaluate the Efficacy and Safety of Boceprevir Added to Standard of Care Therapy in Previously Treated Participants With Chronic Hepatitis C Genotype 1 and Cirrhosis (MK-3034-105) | Hepatitis C Virus (HCV) Infection | Completed | Phase 4 | Merck Sharp & Dohme LLC |
| NCT01544920 | Safety and Efficacy of Boceprevir/Peginterferon Alfa-2a/Ribavirin in Interleukin-28B CC Allele-Positive Chronic Hepatitis C Virus (HCV) Genotype 1 Participants (P07755) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01425190 | Pharmacokinetics of Boceprevir in Pediatric Subjects With Chronic Hepatitis C Genotype 1 (P07614) | Chronic Hepatitis C Virus (HCV) Infection | Terminated (The US FDA and the EU CHMP provided guidance indicating preference for intereferon-free | Phase 1 | Merck Sharp & Dohme LLC |
| NCT01390844 | Safety and Efficacy of Boceprevir in Asia Pacific Participants With Chronic Hepatitis C Genotype 1 (P07063) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT00689390 | Three-year Follow-up of Participants After Administration of Boceprevir or Narlaprevir for the Treatment of Chronic Hepatitis C (P05063) | Chronic Hepatitis C Virus (HCV) Infection / Hepacivirus | Terminated (The study was terminated due to satisfaction of post-marketing commitments) | Phase 2,3 | Merck Sharp & Dohme LLC |
| NCT01353911 | Grazoprevir (MK-5172) Administered With Peginterferon and Ribavirin in Treatment-Naïve Participants With Chronic Hepatitis C (MK-5172-003) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Merck Sharp & Dohme LLC |
| NCT01470690 | Pharmacokinetic Study of the HCV Protease Inhibitor Bo-cePRevir and the Proton Pump Inhibitor OMeprazOle (PROMO) (PROMO) | Gastric Acid-related Disorders / Hepatitis C Virus (HCV) Infection | Completed | Phase 1 | Radboud University Medical Center |
| NCT01590225 | Efficacy and Safety of Boceprevir in Combination With Peginterferon Alfa-2b Plus Ribavirin in Pediatric Subjects With Chronic Hepatitis C Genotype 1 (P08034) | Chronic Hepatitis C Virus (HCV) Infection | Withdrawn | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01288417 | Pharmacokinetic Study of the HCV Protease Inhibitor Boceprevir and the HIV Integrase Inhibitor Raltegravir (OPAL) | Hepatitis C Virus (HCV) Infection/ Human Immunodeficiency Virus Infection(HIV) | Completed | Phase 1 | Radboud University Medical Center |
| NCT01425203 | The Effect of Boceprevir in Russian Participants Diagnosed With Chronic Hepatitis C Genotype 1 (P08160) | Chronic Hepatitis C Genotype 1 | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01023035 | Boceprevir/Peginterferon/Ribavirin for Chronic Hepatitis C: Erythropoietin Use Versus Ribavirin Dose Reduction for Anemia (P06086 AM2) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT00910624 | Boceprevir Treatment in Participants With Chronic Hepatitis C Genotype 1 Deemed Nonresponders to Peginterferon/Ribavirin (P05514) (PROVIDE) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Merck Sharp & Dohme LLC |
| NCT01482767 | Evaluating the Effectiveness of Boceprevir, Pegylated-Interferon Alfa 2b and Ribavirin in Treating Hepatitis C Virus (HCV) Infection in Adults With HIV and HCV Infection | Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT02333292 | Efficacy and Safety of Therapy Against HCV Based on Direct-acting Antivirals in Real-life Conditions | Hepatitis C, Chronic | Completed | Valme University Hospital |