Sequence information
DRAVP ID DRAVPc011
Name Oglufanide(IM862, Glufanide)
Sequence
Molecular Formula C16H19N3O5
Condition/Disease Chronic HCV infection
Group Phase Ⅱclinical trial
Type Peptide
Description Oglufanide is a synthetic dipeptide immunomodulator composed of L-glutamic acid and L-tryptophan joined by a peptide linkage and in development for the treatment of chronic hepatitis C viral infection. Oglufanide was originally developed to treat severe infectious disease in Russia (where it is a registered pharmaceutical), and was extensively studied in cancer clinical trials in the United States before being acquired by Implicit Bioscience in 2005. Oglufanide works as a regulator of the body's immune response, is being given by intranasal administration to patients with chronic hepatitis C viral infection.
Active sequence/Structure
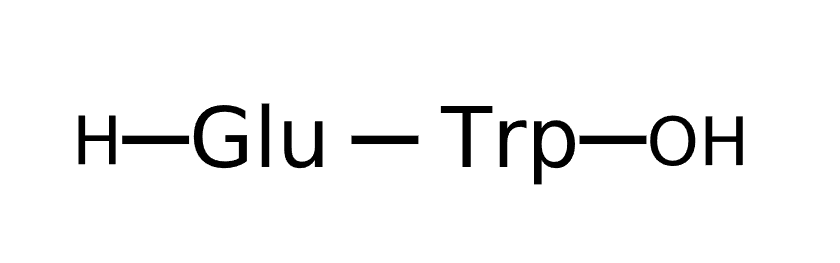
Comment
No comments found.
External Links
DrugBank Accession Number DB05779
Pubchem ID 100094
CHEMBL ID CHEMBL2111029
UNII 4RHY598T5U
CAS 38101-59-6
Reference 34258744
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00002445 | Safety and Effectiveness of an Experimental Drug, IM862, in Treating Kaposi's Sarcoma in AIDS Patients | Human Immunodeficiency Virus (HIV) Infection | Completed | Phase 3 | Cytran |