Sequence information
DRAVP ID DRAVPc025
Name Human interferon beta
Sequence
Molecular Formula C72H115N19O26
Condition/Disease COVID-19
Group Approved
Type Protein
Description Human interferon beta is a polypeptide drug which could used in the treatment of COVID-19. Human interferon beta is a polypeptide used in the management of relapsing forms of Multiple Sclerosis (MS), and was initially approved by the FDA in 1992. Interferon beta is currently being studied as a possible treatment for COVID-19, which results from infection with the novel 2019 SARS-CoV-2 virus.Interferon-beta has been used in the past in clinical studies with other coronaviruses due to its demonstrated activity against the virus causing Middle Eastern Respiratory Syndrome (MERS). It is therefore a potential drug candidate for SARS-CoV-2 based on viral genetic similarity.
Active sequence/Structure
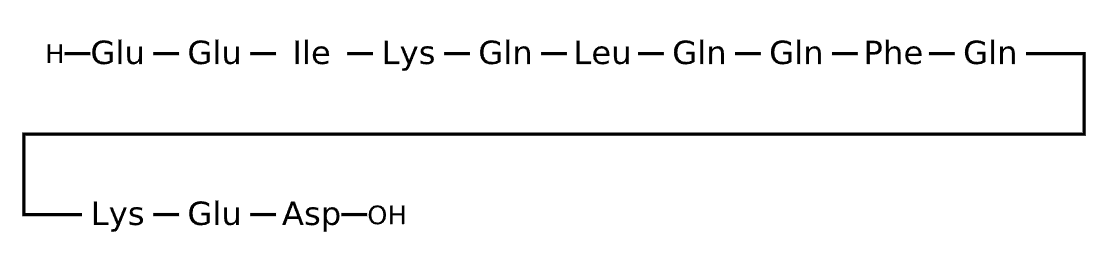
Comment
No comments found.
External Links
DrugBank Accession Number DB14999
Pubchem ID 101632004
CHEMBL ID CHEMBL2108510
UNII V9GU1EM8SF
CAS 74899-71-1
Reference Not Available
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT04324463 | Anti-Coronavirus Therapies to Prevent Progression of Coronavirus Disease 2019 (COVID-19) Trial (ACTCOVID19) | Coronavirus Disease 2019 (COVID‑19) / Severe Acute Respiratory Syndrome (SARS) Treatment | Active, not recruiting | Phase 3 | Population Health Research Institute |