Sequence information
DRAVP ID DRAVPc026
Name Palivizumab
Sequence
Molecular Formula Not Available
Condition/Disease Severe Respiratory Syncytial Virus Infection
Group Approved
Type Antibody
Description Humanized monoclonal antibody (IgG1k) produced by recombinant DNA technology, directed to an epitope in the A antigenic site of the F protein of respiratory syncytial virus (RSV). Synagis is a composite of human (95%) and murine (5%) antibody sequences. It is a monoclonal anti respiratory syncytial virus F protein antibody used to prevent serious sequelae caused by respiratory syncytial virus infection in pediatric patients.
Active sequence/Structure
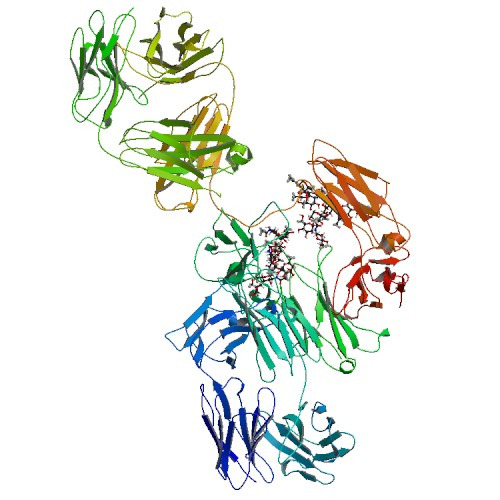
Comment
No comments found.
External Links
DrugBank Accession Number DB00110
Pubchem ID 46506637
CHEMBL ID CHEMBL1201586
UNII DQ448MW7KS
CAS 188039-54-5
Reference Not Available
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02968173 | A Study to Assess the Safety and Effectiveness of Palivizumab Administered to Children at High Risk of Severe Respiratory Syncytial Virus (RSV) Infection in the Russian Federation and the Republic of | Respiratory Syncytial Virus (RSV) Treatment | Completed | Phase 3 | AbbVie |
| NCT01466062 | Clinical Study of Palivizumab in Japanese Newborns, Infants and Young Children at the Age of 24 Months or Less With Immunocompromised Medical Conditions | Infections, Respiratory Syncytial Virus Prevention | Completed | Phase 3 | AbbVie (prior sponsor, Abbott) |