Sequence information
DRAVP ID DRAVPc058
Name Saquinavir
Sequence Not available
Molecular Formula C38H50N6O5
Condition/Disease HIV infection
Group Approved
Type Peptidomimetic
Description Saquinavir is an aspartic acid derivative obtained by formal condensation of the primary amino group of (2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarbamoyl)octahydroisoquinolin-2(1H)-yl]-3-hydroxy-1-phenylbutan-2-ylamine with the carboxy group of N(2)(-quinolin-2-ylcarbonyl)-L-asparagine. It is a member of quinolines and a L-asparagine derivative. Saquinavir is an HIV-1 protease inhibitor used in combination with ritonavir and other antiretrovirals for the Treatment of human immunodeficiency virus-1(HIV-1).
Active sequence/Structure
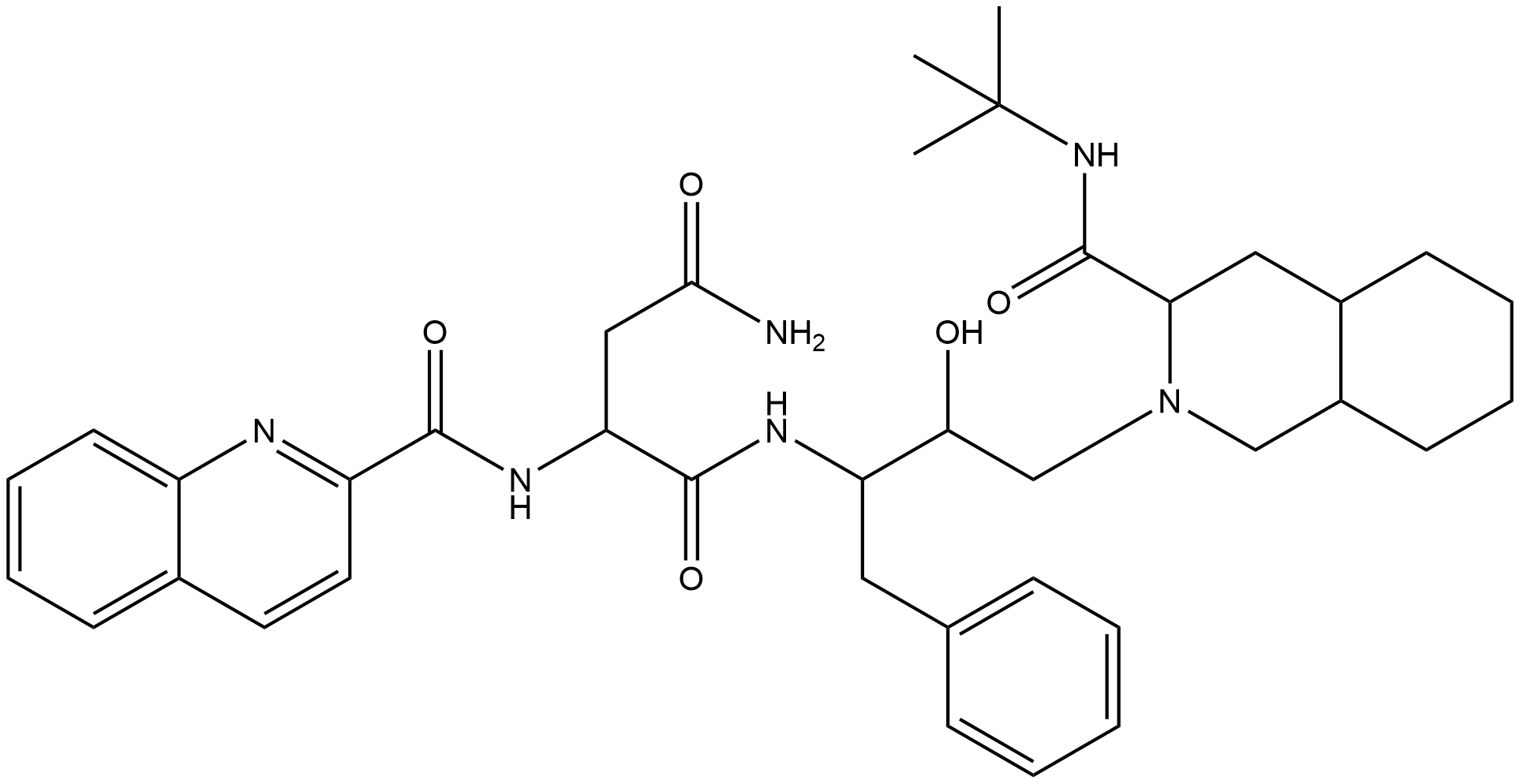
Comment
No comments found.
External Links
DrugBank Accession Number DB01232
Pubchem ID 441243
CHEMBL ID CHEMBL114
UNII L3JE09KZ2F
CAS 127779-20-8
Reference 36466430
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00002229 | Safety and Effectiveness of Adding Saquinavir (FORTOVASE) in Soft Gel Capsule Form to an Anti-HIV Drug Combination in HIV-Infected Patients | HIV Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT00379405 | Saquinavir/Ritonavir in Single Therapy as Maintenance Treatment | HIV Infections | Completed | Phase 4 | Germans Trias i Pujol Hospital |
| NCT00002367 | A Study of Saquinavir Soft Gelatin Capsules Plus Zidovudine Plus Lamivudine in the Treatment of HIV-1 Infected Patients Who Have Never Taken Anti-HIV Drugs | HIV Infections | Completed | Phase 3 | Hoffmann-La Roche |
| NCT00002382 | A Study of Saquinavir Used Alone or in Combination With Other Anti-HIV Drugs in HIV-Infected Patients | HIV Infections | Completed | Phase 3 | Hoffmann-La Roche |