Sequence information
DRAVP ID DRAVPc003
Name Plitidepsin(Aplidine)
Sequence
Molecular Formula C36H53N7O6
Condition/Disease Chronic HCV infection
Group Phase Ⅲ clinical trial
Type Peptide
Description Plitidepsin is a didemnin that is didemin B in which the hydroxy group of the 1-(2-hydroxypropanoyl)-L-prolinamide moiety has been oxidised to the corresponding ketone. It was originally isolated from the Mediterranean tunicate Aplidium albicans. It has a role as a marine metabolite, an antineoplastic agent and an anticoronaviral agent.
Active sequence/Structure
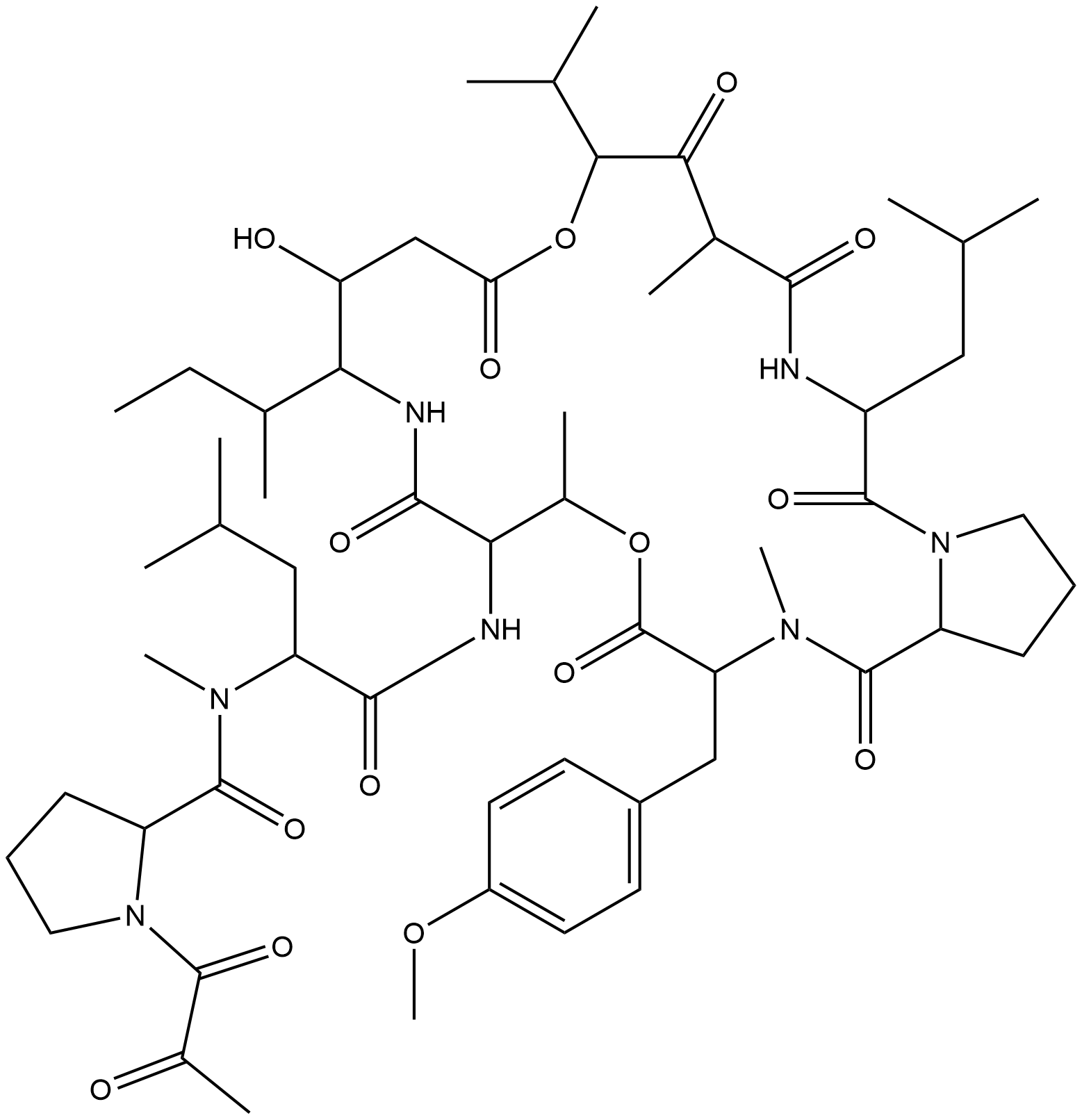
Comment
No comments found.
External Links
DrugBank Accession Number DB05521
Pubchem ID 3010818
CHEMBL ID CHEMBL451930
UNII Y76ID234HW
CAS 137219-37-5
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT01336829 | TMC125IFD1001 - Drug-Drug Interaction of Etravirine With Telaprevir and TMC278 With Telaprevir. | Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 1 | Tibotec Pharmaceuticals, Ireland |
| NCT01563328 | A Study to Evaluate the Effect of Boceprevir and Telaprevir on Dolutegravir Pharmacokinetics in Healthy Adult Subjects (ING115697). | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 1 | ViiV Healthcare |
| NCT01332955 | Telaprevir in HIV-HCV Coinfected Patients Who Had Previously Failed a Peginterferon-Ribavirin Regimen | Chronic Hepatitis C Virus (HCV) Infection / Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 2 | ANRS, Emerging Infectious Diseases |
| NCT01581138 | VX-222 + Telaprevir + Ribavirin for 12 or 16 Weeks in Treatment-Naive Subjects With Genotype 1a Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Vertex Pharmaceuticals Incorporated |
| NCT01516918 | A Study to Evaluate the Efficacy and Safety of Quadruple Therapy (VX-222, Telaprevir,Peginterferon-Alfa-2a, Ribavirin) in Subjects With Chronic Hepatitis C With Compensated Cirrhosis | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Vertex Pharmaceuticals Incorporated |
| NCT00528528 | An Open-Label Study of Telaprevir Administered Every 12 or 8 Hours in Combination With One of Two Pegylated Interferons and Ribavirin in Treatment-Naive Genotype 1 Chronic Hepatitis C Participants | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Tibotec BVBA |
| NCT00372385 | Phase 2 Study of VX-950, Pegasys® With and Without Copegus® in Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase2 | Vertex Pharmaceuticals Incorporated |
| NCT00336479 | Phase 2 Study of VX-950, Pegasys®, and Copegus® in Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Vertex Pharmaceuticals Incorporated |
| NCT01994486 | Open-Label Safety Study of Telaprevir and Sofosbuvir in Chronic Hepatitis C Genotype 1 | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | University of Florida |
| NCT01648140 | Dose Ranging of GSK2336805 in Combination Therapy | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | GlaxoSmithKline |
| NCT00561015 | A Phase 2a Study to Evaluate Viral Kinetics and Safety of Telaprevir in Participants With Genotype 2 or 3 Hepatitis C Infection | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Tibotec BVBA |
| NCT00262483 | Phase 2 Study of VX-950, Pegasys and Copegus in Hepatitis C | Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Vertex Pharmaceuticals Incorporated |
| NCT01858961 | Open Label Trial to Compare BI 207127 to Telaprevir in HCV Patients | Chronic Hepatitis C Virus (HCV) Infection | Withdrawn | Phase 3 | Boehringer Ingelheim |
| NCT01467479 | A Study to Treat Subjects With Telaprevir, Ribavirin, and Peginterferon Who Are Coinfected With HIV and Hepatitis C Virus (HCV) | Hepatitis C Virus (HCV) Infection | Terminated (It was decided by Sponsor on 13 January 2014 to terminate study early at primary efficac | Phase 3 | Vertex Pharmaceuticals Incorporated |
| NCT01459913 | Efficacy of a 12-Week Regimen of Telaprevir, Pegylated Interferon, and Ribavirin in Treatment-Naive and Prior Relapser Subjects With Interleukin28B (IL28B) CC Genotype | Chronic Hepatitis C Virus (HCV) Infection | Terminated (The study was terminated early by the sponsor on 13 January 2014 due to a decision to mo | Phase 3 | Vertex Pharmaceuticals Incorporated |
| NCT01718145 | A Phase 3, Comparative Study of Asunaprevir and Daclatasvir Combination Therapy Versus Telaprevir Therapy in Japanese HCV Subjects | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Bristol-Myers Squibb |
| NCT01598090 | Phase 3 Efficacy and Safety Study of Peginterferon Lambda-1a and Ribavirin With Telaprevir | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Bristol-Myers Squibb |
| NCT01718158 | Efficacy and Safety Evaluation of a Regimen Consisting of Peginterferon Lambda-1a + Ribavirin + Daclatasvir (Lambda + RBV + DCV) in HCV Genotype 1b Treatment naïve Patients or Prior Relapsers to Pegin | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Bristol-Myers Squibb |
| NCT01492426 | Study Comparing Daclatasvir (BMS-790052) With Telaprevir Combined With Peginterferon Alfa-2a and Ribavirin in Patients With Chronic Hepatitis C Virus Infection | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Bristol-Myers Squibb |
| NCT00781274 | Efficacy and Safety of MP-424, Peginterferon Alfa-2b and Ribavirin in Chronic Hepatitis C Who Have Not Achieved an Undetectable HCV RNA Level With Previous Interferon Based Therapy | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT00780416 | Efficacy and Safety of MP-424/Peginterferon Alfa-2b/Ribavirin Combination in Treatment-Naïve Patients With Chronic Hepatitis C | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT00627926 | A Phase 3 Study of Telaprevir in Combination With Pegasys® and Copegus® in Treatment-Naive Subjects With Genotype 1 Hepatitis C Virus (HCV) | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Vertex Pharmaceuticals Incorporated |
| NCT00758043 | A Study Evaluating 24-Week and 48-Week Telaprevir-Based Regimen in Treatment Naïve Subjects With Genotype 1 Chronic Hepatitis C Who Achieve an Extended Rapid Viral Response | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Vertex Pharmaceuticals Incorporated |
| NCT01753557 | Efficacy and Safety of MP-424, Peginterferon Alfa-2a (PEG-IFN Alfa-2a), and Ribavirin(RBV) in Treatment-Naïve or Relapsed Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT01753570 | Efficacy and Safety of MP-424, Interferon Beta (IFN Beta), and Ribavirin(RBV) in Treatment-Naïve or Having Received Interferon Based Therapy With Chronic Hepatitis C (CHC) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT01854697 | A Study to Evaluate the Efficacy and Safety of Three Experimental Drugs Compared With Telaprevir (a Licensed Product) in People With Hepatitis C Virus Infection Who Have Not Had Treatment Before | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | AbbVie |
| NCT01854528 | A Study to Evaluate the Efficacy and Safety of Three Experimental Drugs Compared With Telaprevir (a Licensed Product) for Treatment of Chronic Hepatitis C Infection in Treatment-experienced Adults | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | AbbVie |
| NCT01513941 | An Efficacy and Safety Study of Telaprevir in Patients Infected With Both Chronic Hepatitis C Virus (HCV-1) and Human Immunodeficiency Virus (HIV-1) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Janssen-Cilag International NV |
| NCT01468584 | Efficacy and Safety of MP-424, Peginterferon Alfa-2b and Ribavirin in Non-responder Genotype 2 Hepatitis C Infected Patients | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT01466192 | Efficacy and Safety of MP-424, Peginterferon Alfa-2b and Ribavirin in Relapser Genotype 2 Hepatitis C Infected Patients | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT00780910 | Efficacy and Safety of MP-424, Peginterferon Alfa-2b, and Ribavirin in Patients With Chronic Hepatitis C Who Relapsed After Previous Interferon Based Therapy | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Mitsubishi Tanabe Pharma Corporation |
| NCT01500616 | Telaprevir Open-Label Study in Co-Infected Patients | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Janssen-Cilag International NV |
| NCT01054573 | VX-950-TiDP24-C219: A Roll Over Trial for Patients in the Control Group of the C216 Study Who Received Telaprevir Placebo | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Janssen Infectious Diseases BVBA |
| NCT00703118 | A Safety and Effectiveness Study of Telaprevir in Chronic, Genotype 1, Hepatitis C Patients That Failed Previous Standard Treatment | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Tibotec BVBA |
| NCT01571583 | An Efficacy and Safety Study of Telaprevir in Patients With Genotype 1 Hepatitis C Infection After Liver Transplantation | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Janssen-Cilag International NV |
| NCT01241760 | VX-950-C211 - A Dosing Regimen Study (Twice Daily Versus Every 8 Hours) of Telaprevir in Treatment-naïve Participants With Genotype 1 Chronic Hepatitis C Virus Infection | Chronic Hepatitis C Genotype 1 | Completed | Phase 3 | Janssen Infectious Diseases BVBA |
| NCT01498068 | Open-Label, Bridging Study of Telaprevir in Treatment-Naïve and Treatment-Experienced Russian Patients With Genotype 1 Chronic Hepatitis C | Chronic Hepatitis C Genotype 1 | Completed | Phase 3 | Janssen-Cilag International NV |
| NCT02006745 | Open Label Trial of PEG-IFN, RBV & TVR vs. PEG-IFN & RBV Alone in Tx of HCV-1 in HIV-1 Co-infected Patients (CHAT) (CHAT) | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | St Stephens Aids Trust |
| NCT01415141 | Peginterferon and Ribavirin, With or Without Telaprevir, for Genotype 1 Hepatitis C and IL28B CC Polymorphism | Hepatitis C Virus (HCV) Infection | Withdrawn (Lack of funding) | Phase 4 | University of Vermont |
| NCT02087111 | Telaprevir in Genotype 3 HCV (TIG3) | Hepatitis C Virus (HCV) Infection | Unknown | Phase 4 | Queen Mary University of London |
| NCT01766115 | Hepatitis C Virus Post-Exposure Prophylaxis for Health Care Workers | Hepatitis C Virus (HCV) Infection | Withdrawn | Phase 4 | Massachusetts General Hospital |
| NCT01592006 | Pegylated Interferon, Ribavirin, Telaprevir in Hepatitis C Virus Infection in Orthotopic Liver Transplant Recipients | Hepatitis C Virus (HCV) Infection | Terminated (Low accrual) | Phase 4 | University of Chicago |
| NCT01467492 | Telaprevir, Peg-IFN-alfa-2a, and RBV in Treatment-Experienced Black/African American and Non-Black/African American Subjects With Genotype 1 Chronic Hepatitis C | Hepatitis C Virus (HCV) Infection | Terminated (It was decided by Sponsor on 13 January 2014 to terminate study early at primary efficac | Phase 4 | Vertex Pharmaceuticals Incorporated |
| NCT01743521 | DAA Based Therapy for Recently Acquired Hepatitis C (DARE-C) (DARE-C) | Chronic Hepatitis C Virus (HCV) Infection | Completed | Phase 4 | Kirby Institute |