Sequence information
DRAVP ID DRAVPc004
Name Enfuvirtide(Fuzeon, DP178)
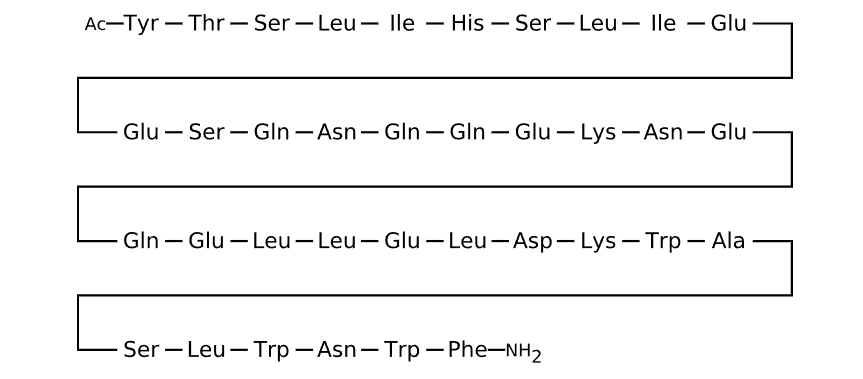
Sequence
Molecular Formula C204H301N51O64
Condition/Disease HIV Infections
Group Approved
Type Peptide
Description Enfuvirtide is a 36 amino acid biomimetic peptide that is structurally similar to the HIV proteins that are responsible for the fusion of the virus to cell membranes and subsequent intracellular uptake. The first agent in the novel class of antiretroviral drugs called HIV fusion inhibitors, enfuvirtide works by inhibiting HIV-1 fusion with CD4 cells. It binds to the first heptad-repeat (HR1) in the gp41 subunit of the viral envelope glycoprotein and prevents the conformational changes required for the fusion of viral and cellular membranes. By disrupting the HIV-1 molecular machinery during its final stage of fusion with the target cell, enfuvirtide limits the spread of further infection.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB00109
Pubchem ID 16130199
CHEMBL ID CHEMBL525076
UNII 19OWO1T3ZE
CAS 159519-65-0
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00022763 | T-20 Plus a Selected Anti-HIV Treatment in HIV-Infected Children and Adolescents | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 2 | Hoffmann-La Roche |
| NCT00002228 | A Study of T-20 in HIV-Positive Adults | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 2 | Trimeris |
| NCT02733419 | Efficacy, and Safety Study of Optimized Background Antiretroviral Regimen (OB) in Combination With Enfuvirtide in the Treatment-Experienced Participants With Human Immunodeficiency Virus-1 (HIV-1) Inf | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | Hoffmann-La Roche |
| NCT00454337 | Efficacy and Tolerance of the Switch From Enfuvirtine to Raltegravir in Antiretroviral Therapy Regimen in HIV Patients With Undetectable Viral Load | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | French National Agency for Research on AIDS and Viral Hepatitis |
| NCT00050856 | Fuzeon (Enfuvirtide) Early Access Program for Patients With HIV-1 Infection | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT00008528 | T-20 With Anti-HIV Combination Therapy for Patients With Prior Anti-HIV Drug Treatment and/or Drug Resistance to Each of the Three Classes of Approved Anti-HIV Drugs | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | Hoffmann-La Roche |
| NCT02582983 | A Study of Enfuvirtide (Fuzeon) in Participants With Advanced Human Immunodeficiency Virus (HIV) Infection | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT02569502 | A Study of Enfuvirtide (Fuzeon) in Patients With Advanced Human Immunodeficiency Virus-1 (HIV-1) Infection | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT00487188 | A Study to Evaluate the Safety and Efficacy of Adding Enfuvirtide to Oral Highly Active Antiretroviral Therapy (HAART) in Human Immunodeficiency Virus (HIV) Patients With Prior Treatment Experience | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT00337701 | BOSS Study: A Study of Fuzeon Using the Needle-Free Biojector 2000 in Patients With HIV-1 Infection | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Rochev |
| NCT00326963 | BLQ Study: A Study of a Protease Inhibitor With Fuzeon (Enfuvirtide) in Treatment-Experienced Patients With HIV-1. | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT00233883 | WAND Study - A Study to Evaluate Fuzeon (Enfuvirtide) Administered by a Needle-Free Injection Device in Patients With HIV. | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT00344760 | A Study to Evaluate of the Efficacy of Enfuvirtide During the Induction Phase of Therapy | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | University of Maryland, Baltimore |
| NCT00232908 | QUALITE Study - A Study of Fuzeon (Enfuvirtide) in Treatment-Experienced Patients With Human Immunodeficiency Virus-1 (HIV-1) Infection | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 4 | Hoffmann-La Roche |
| NCT00021554 | T-20 in HIV Patients With Prior Drug Treatment and/or Resistance to Each of the Three Classes of Anti-HIV Drugs | Human Immunodeficiency Virus (HIV) Infections | Completed | Phase 3 | Hoffmann-La Roche |