Sequence information
DRAVP ID DRAVPc007
Name Albuvirtide
Sequence
Molecular Formula C204H306N54O72
Condition/Disease HIV infection
Group Phase Ⅲ clinical trial(HCV infection treatment), Phase Ⅱ clinical trial(COVID-20)
Type Peptide
Description Albuvirtide (ABT) belongs to the class of HIV-1 fusion inhibitors. It is a 3-maleimimidopropionic acid (MPA)-modified peptide derived from the C-terminal heptad repeat sequence of HIV-1 gp41. It binds to gp41, blocking HIV-1 from entering and infecting certain cells
Active sequence/Structure
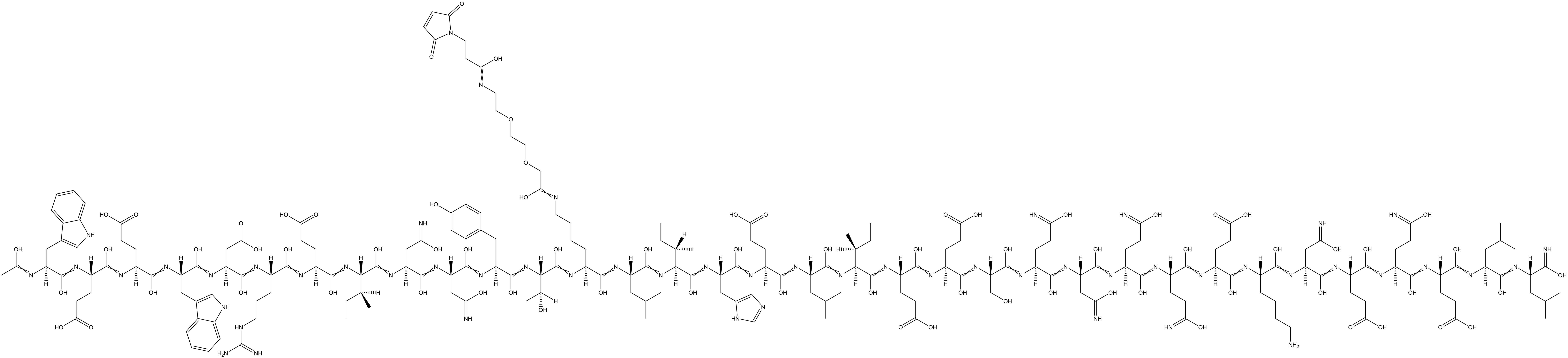
Comment
X is a 3-maleimimidropropionic acid(MPA) modification.
External Links
DrugBank Accession Number Not Available
Pubchem ID 134694278
CHEMBL ID CHEMBL4297257
UNII MG9PC54E43
CAS 1417179-66-8
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02369965 | Test Albuvirtide in Experienced Patients (TALENT) | HIV Infections/AIDS | Completed | Phase 3 | Frontier Biotechnologies Inc. |
| NCT04819347 | Albuvirtide in Combination With 3BNC117 in Virologically Suppressed Subjects With HIV-1 Infection | Human Immunodeficiency Virus (HIV) Infections/AIDS | Not yet recruiting | Phase 2 | Frontier Biotechnologies Inc. |
| NCT04560569 | Albuvirtide in Combination With 3BNC117 in Patients With Multi-Drug Resistant (MDR) HIV-1 Infection | Human Immunodeficiency Virus (HIV) Infections/AIDS | Unknown | Phase 2 | Frontier Biotechnologies Inc. |