Sequence information
DRAVP ID DRAVPc008
Name Alisporivir(Debio-025)
Sequence
Molecular Formula C63H113N11O12
Condition/Disease Chronic HCV infection, COVID-19
Group Phase Ⅲ clinical trial(HCV infection treatment), Phase Ⅱ clinical trial(COVID-19)
Type Peptide
Description Alisporivir is a non-immunosuppressive analogue of cyclosporine A and an inhibitor of cyclophilins, with potential antiviral activity. Upon oral administration, alisporivir targets and inhibits human host cyclophilins, thereby inhibiting hepatitis C virus (HCV) replication in hepatocytes. Alisporivir may also inhibit the replication of various coronaviruses. In addition, it may inhibit mitochondrial cyclophilin-D, which regulates mitochondrial permeability transition pore (mPTP) opening. This may prevent cell death and tissue damage. It has a role as an anticoronaviral agent.
Active sequence/Structure
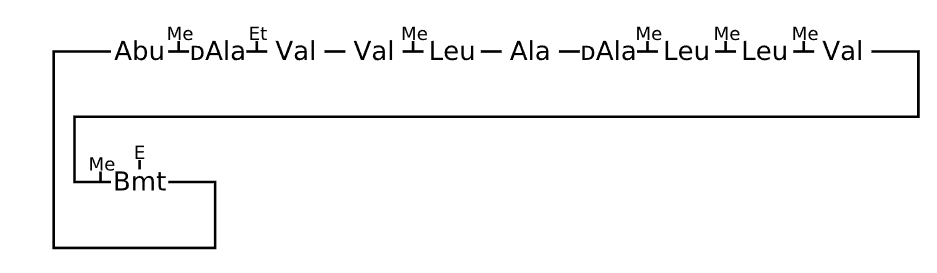
Comment
The first X stands for D-Aminobutyric acid.The second X stands for Beta-methyl-tyrosine.
External Links
DrugBank Accession Number DB12139
Pubchem ID 11513676
CHEMBL ID CHEMBL1651956
UNII VBP9099AA6
CAS 254435-95-5
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00537407 | A Study of Debio 025 in Combination With PegIFN Alpha-2a and Ribavirin in Chronic HCV Patients Non-responders to Standard Treatment | Chronic Hepatitis C virus infection | Completed | Phase 2 | Debiopharm International SA |
| NCT01500772 | Alisporivir With PEG and RBV in Protease Inhibitor (PI) Treatment Failure Patients With Chronic Hepatitis C | Hepatitis C Virus (HCV) Infection | Terminated | Phase 3 | Debiopharm International SA |
| NCT01183169 | Efficacy and Safety of Adding Alisporivir (DEB025) to Peginterferon (IFN) Alfa-2a (Peg-IFN Alfa-2a) and Ribavirin in Chronic HCV Genotype 1 Patients Who Relapsed or Did Not Respond to Previous Treatme | Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Debiopharm International SA |
| NCT01215643 | Efficacy and Safety of Alisporivir Alone or Combined With RBV or PEG in Chronic Hepatitis C Genotype 2 and 3 Treatment-naïve Participants | Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Debiopharm International SA |
| NCT02753699 | Long Term Follow-up Study to Assess Durability of Sustained Virologic Response in Alisporivir-treated Hepatitis C Patients | Hepatitis C Virus (HCV) Infection | Completed | Phase 3 | Debiopharm International SA |
| NCT01318694 | Efficacy and Safety of Alisporivir Triple Therapy in Chronic Hepatitis C Genotype 1 Treatment-naïve Participants | Chronic Hepatitis C virus infection | Completed | Phase 3 | Debiopharm International SA |
| NCT02094443 | Alisporivir With RBV in Chronic Hepatitis C Genotype 2 and 3 Participants for Whom Interferon is Not an Option | Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Debiopharm International SA |
| NCT01446250 | Alisporivir (Deb025) and Boceprevir Triple Therapies in African American Participants Not Previously Treated for Chronic Hepatitis C Genotype 1 | Hepatitis C Virus (HCV) Infection | Terminated | Phase 3 | Debiopharm International SA |
| NCT04608214 | Evaluation of Alisporivir for the Treatment of Hospitalised Patients With Infections Due to SARS-CoV-2 (COVID-19) | COVID-19 | Not yet recruiting | Phase 2 | Assistance Publique - Hôpitaux de Paris |