Sequence information
DRAVP ID DRAVPc009
Name Thymalfasin
Sequence
Molecular Formula C129H215N33O55
Condition/Disease HBV infection, HCV infection
Group Approved
Type Peptide
Description A thymus hormone polypeptide found in thymosin fraction 5 (a crude thymus gland extract) but now produced by synthesis.Thymosin alpha 1 is now approved in 35 developing countries for the treatment of Hepatitis B and C. Thymalfasin is also used for the treatment of chemotherapy-induced immunosuppression, and to enhance the efficacy of influenza and hepatitis B vaccines in immunocompromised patients.
Active sequence/Structure
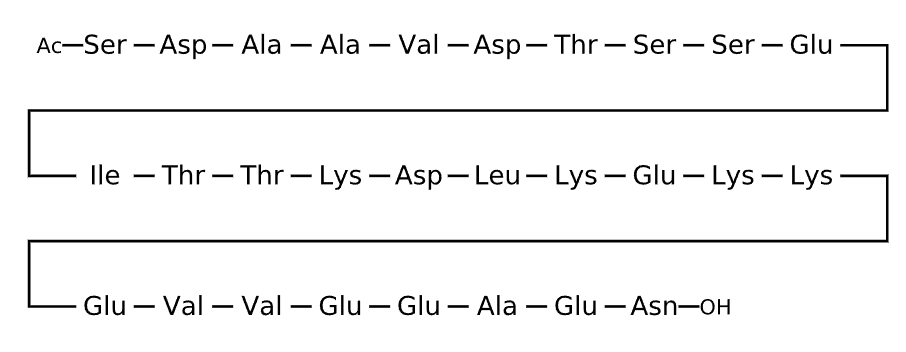
Comment
No comments found.
External Links
DrugBank Accession Number DB04900
Pubchem ID 16130571
CHEMBL ID CHEMBL2103979
UNII W0B22ISQ1C
CAS 62304-98-7
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02281266 | Thymalfasin Adjuvant Therapy in Hepatitis B Virus (HBV)-Related Hepatocellular Carcinoma (HCC) After Curative Resection | Curable Hepatitis B Virus-Related Hepatocellular Carcinoma | Unknown | Phase 4 | Jia Fan |
| NCT03448744 | Efficacy and Safety of Combination Therapy of Thymalfasin and Entecavir in HBeAg-positive ETV-experienced Patients | Chronic Hepatitis B virus infection | Unknown | Phase 4 | Wen-hong Zhang |
| NCT04428008 | Thymosin Alpha 1 to Prevent COVID-19 Infection in Renal Dialysis Patients (Ta1) | COVID-19 | Recruiting | Phase 2 | Inova Health Care Services |
| NCT04487444 | Thymalfasin (Thymosin Alpha 1) to Treat COVID-19 Infection (Ta1) | COVID-19 | Recruiting | Phase 2 | Rhode Island Hospital |