Sequence information
DRAVP ID DRAVPc012
Name Telaprevir
Sequence Not available
Molecular Formula C36H53N7O6
Condition/Disease Chronic HCV infection
Group Approved, Withdrawn
Type Peptidomimetic
Description Telaprevir is an oligopeptide consisting of N-(pyrazin-2-ylcarbonyl)cyclohexylalanyl, 3-methylvalyl, octahydrocyclopenta[c]pyrrole-1-carboxy, and 3-amino-N-cyclopropyl-2-oxohexanamide residues joined in sequence. Telaprevir is an orally available peptidomimetic small molecule with activity against hepatitis C virus (HCV). Telaprivir is a selective protease inhibitor that targets the viral HCV NS3-4A serine protease and disrupts processing of viral proteins and formation of a viral replication complex.
Active sequence/Structure
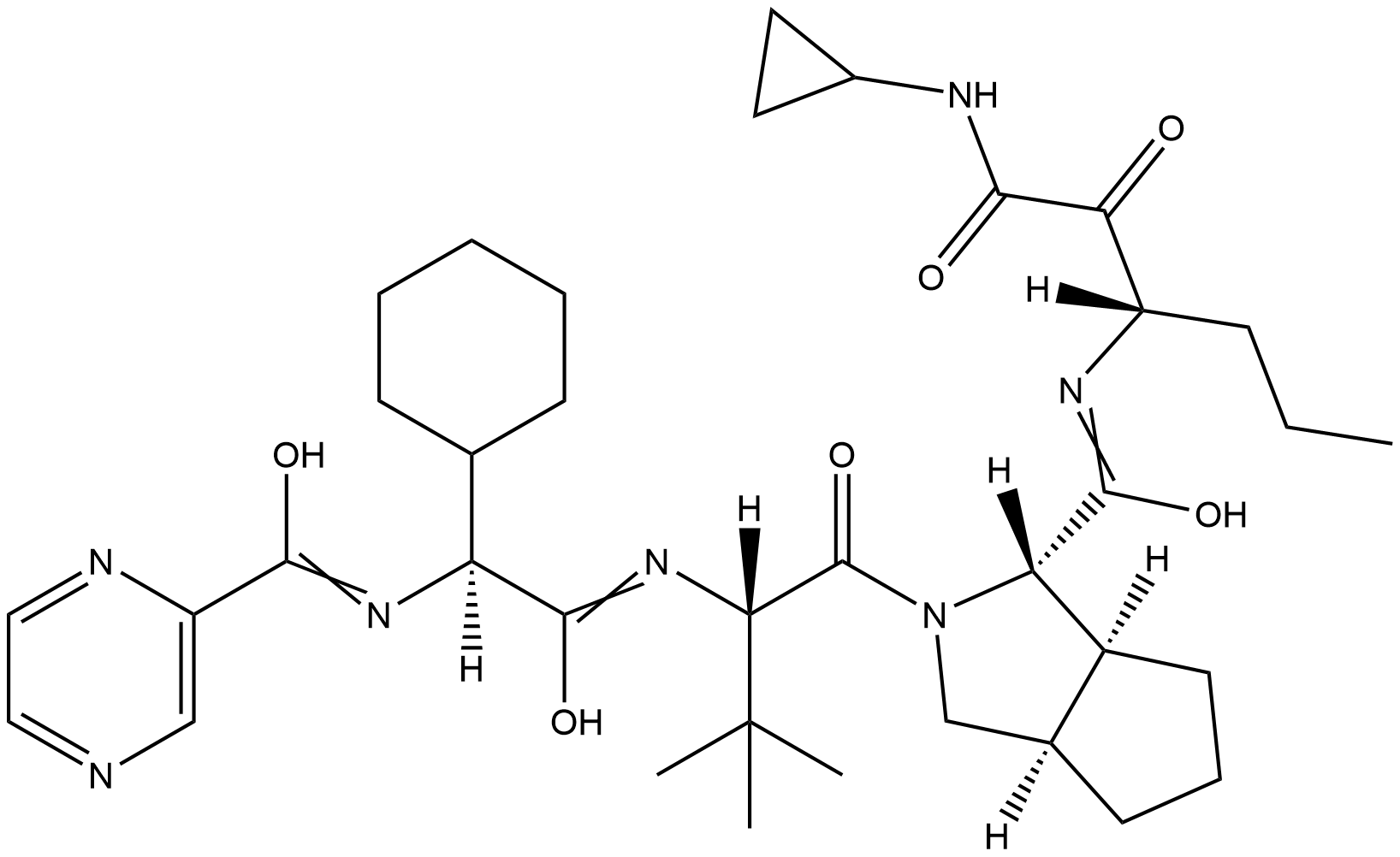
Comment
No comments found.
External Links
DrugBank Accession Number DB05521
Pubchem ID 3010818
CHEMBL ID CHEMBL231813
UNII 655M5O3W0U
CAS 402957-28-2
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT01766167 | Clinical Pharmacology Study of MP-424 | Chronic Hepatitis C virus infection | Completed | Phase 1 | Mitsubishi Tanabe Pharma Corporation |
| NCT00621296 | Safety and Efficacy of MP-424 to Treat Chronic Hepatitis C | Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | Mitsubishi Tanabe Pharma Corporation |
| NCT00591214 | Safety and PK Study of MP-424 to Treat Chronic Hepatitis C | Chronic Hepatitis C virus infection | Completed | Phase 1 | Mitsubishi Tanabe Pharma Corporation |
| NCT00983853 | Safety and Efficacy of Telaprevir in Combination With Peginterferon Alfa-2a and Ribavirin in Subjects Co-Infected With Hepatitis C Virus (HCV) and HIV | Hepatitis C Virus (HCV) Infection/Human Immunodeficiency Virus (HIV) Infection | Completed | Phase 2 | Vertex Pharmaceuticals Incorporated |
| NCT00251199 | VX-950 and Peginterferon for Hepatitis C | Hepatitis C Virus (HCV) Infection | Completed | Phase 1 | Vertex Pharmaceuticals Incorporated |