Sequence information
DRAVP ID DRAVPc013
Name Atazanavir
Sequence Not available
Molecular Formula C38H52N6O7
Condition/Disease HIV infection
Group Approved
Type Peptidomimetic
Description Atazanavir is an aza-dipeptide analogue with a bis-aryl substituent on the (hydroxethyl)hydrazine moiety. Atazanavir is an antiretroviral protease inhibitor that is used in the therapy and prevention of human immunodeficiency virus (HIV-1) infection and the acquired immunodeficiency syndrome (AIDS). The U.S. Food and Drug Administration (FDA) approved atazanavir on June 20, 2003.
Active sequence/Structure
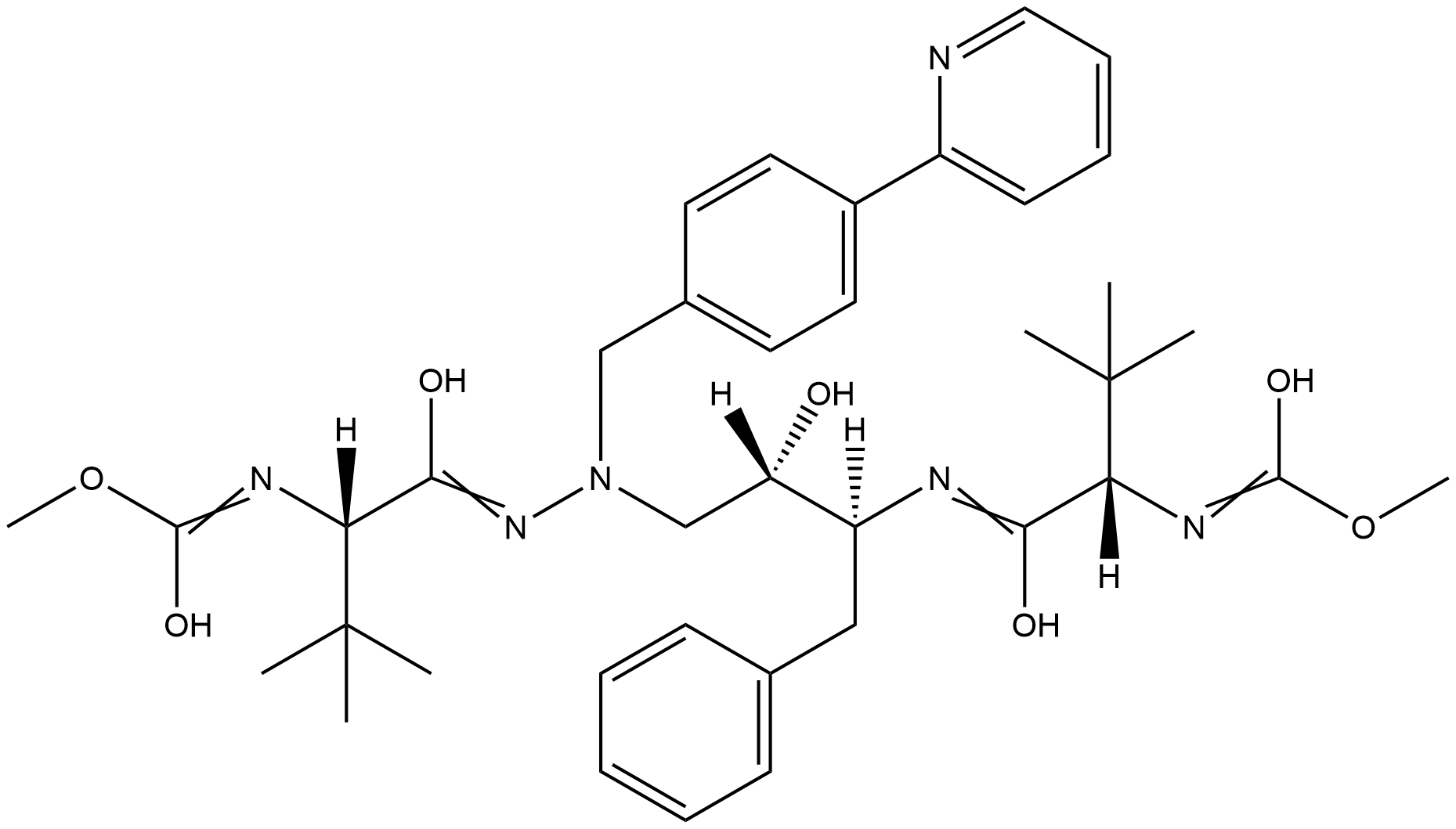
Comment
No comments found.
External Links
DrugBank Accession Number DB01072
Pubchem ID 148192
CHEMBL ID CHEMBL1163
UNII QZU4H47A3S
CAS 198904-31-3
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT04459286 | The Nitazoxanide Plus Atazanavir for COVID-19 Study (NACOVID) | COVID-19 | Terminated (IDSMB recommendation) | Phase 2 | Obafemi Awolowo University |
| NCT00312754 | A Phase IV Study of BMS-232632 in HIV+ Patients With Metabolic Syndrome | Human Immunodeficiency Virus (HIV) Infection | Terminated (insufficient enrollment) | Phase 4 | Bristol-Myers Squibb |
| NCT01388543 | Genetics and HIV-1 Protease Inhibitors | Human Immunodeficiency Virus (HIV) Infection | Completed | Phase 4 | University of Colorado, Denver |