Sequence information
DRAVP ID DRAVPc017
Name Peginterferon alfa-2b
Sequence
Molecular Formula Not Available
Condition/Disease Chronic HCV infection
Group Approved
Type Protein
Description Peginterferon alfa-2b is a form of recombinant interferon used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV).It is approved in 2001 by the FDA, Pegintron is indicated for the treatment of HCV with Ribavirin or other antiviral drugs.
Active sequence/Structure
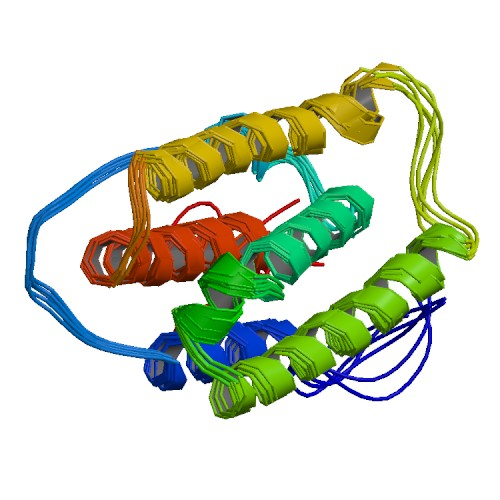
Comment
No comments found.
External Links
DrugBank Accession Number DB00022
Pubchem ID 46506669
CHEMBL ID CHEMBL1201561
UNII G8RGG88B68
CAS 215647-85-1
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02247440 | HCV-HIV Co-infected Patient Cohort in Thailand | Hepatitis C Infections / Human Immunodeficiency Virus (HIV) Infections Treatment | Completed | Phase 4 | Institut de Recherche pour le Developpement |
| NCT01532843 | Lowering Viral Load With Nucleos(T)Ide Analogues Prior to Peginterferon Treatment to Ncrease Sustained Response in CHB (PEGON) | Hepatitis B Chronic Infection Treatment | Completed | Phase 4 | Foundation for Liver Research |
| NCT01838772 | HCV Treatment in HIV Co-Infected Patients in Asia | Human Immunodeficiency Virus (HIV) Infections / Hepatitis C Virus (HCV) Infection Treatment | Completed | Phase 4 | amfAR, The Foundation for AIDS Research |
| NCT00724620 | PEG-IFN Alfa-2b Plus Ribavirin for Treatment of Mexican naïve Patients With Chronic Hepatitis C Infected With Genotype 1 (Study P04511)(COMPLETED) | Chronic Hepatitis C Virus (HCV) Infection Treatment | Completed | Phase 4 | Schering-Plough |
| NCT02168361 | The SIM-SOF Trial for Hepatitis C | Chronic Hepatitis C Virus (HCV) Infection Treatment | Completed | Phase 4 | Center For Hepatitis C, Atlanta, GA |