Sequence information
DRAVP ID DRAVPc023
Name Aldesleukin
Sequence
Molecular Formula C690H1115N177O202S6
Condition/Disease HIV infection, COVID-19
Group Approved
Type Protein
Description Aldesleukin is a recombinant analog of interleukin-2 used to induce an adaptive immune response in the treatment of renal cell carcinoma.Aldesleukin binds to the IL-2 receptor which leads to heterodimerization of the cytoplasmic domains of the IL-2R beta and gamma(c) chains, activation of the tyrosine kinase Jak3, and phosphorylation of tyrosine residues on the IL-2R beta chain. These events led to the creation of an activated receptor complex, to which various cytoplasmic signaling molecules are recruited and become substrates for regulatory enzymes (especially tyrosine kinases) that are associated with the receptor. These events stimulate growth and differentiation of T cells.
Active sequence/Structure
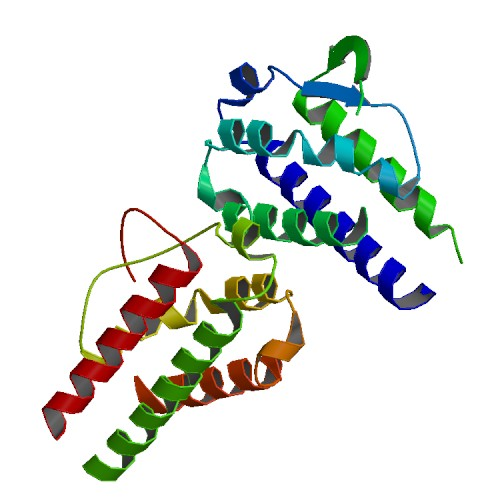
Comment
No comments found.
External Links
DrugBank Accession Number DB00041
Pubchem ID 46508054
CHEMBL ID CHEMBL1201438
UNII M89N0Q7EQR
CAS 110942-02-4
Reference Not Available
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00004978 | An International Study to Evaluate Recombinant Interleukin-2 in HIV Positive Patients Taking Anti-retroviral Therapy (ESPRIT) | Human Immunodeficiency Virus (HIV) Infections Treatment | Completed | Phase 3 | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT00013611 | Interleukin-2 Plus Antiretroviral Therapy for HIV-Infected Patients With Low CD4+ Counts (SILCAAT Study) (SILCAAT) | Human Immunodeficiency Virus (HIV) Infections Treatment | Completed | Phase 3 | University of Minnesota |
| NCT04724629 | Survival TRial Using CytoKines in COVID-19 (STRUCK Trial) (STRUCK) | Coronavirus Disease 2019 (COVID‑19) Treatment | Completed | Phase 3 | University of Sao Paulo |