Sequence information
DRAVP ID DRAVPc053
Name Angiotensin II
Sequence
Molecular Formula C50H71N13O12
Condition/Disease COVID-19
Group Approved
Type Peptide
Description Angiotensin II is a peptide hormone of the RAAS system used to raise blood pressure in septic or other forms of shock. As of December 21, 2017 the FDA approved La Jolla Pharmaceutical's Giapreza (angiotensin II) Injection for Intravenouse Infusion for the indication of acting as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. It is under investigation in clinical trial for the treatment of COVID-19.
Active sequence/Structure
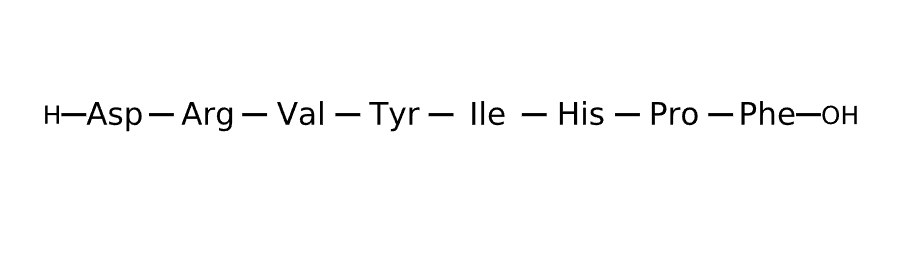
Comment
No comments found.
External Links
DrugBank Accession Number DB11842
Pubchem ID 172198
CHEMBL ID CHEMBL408403
UNII M089EFU921
CAS 4474-91-3
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT04394117 | Controlled evaLuation of Angiotensin Receptor Blockers for COVID-19 respIraTorY Disease (CLARITY) | COVID-19 | Completed | Phase 4 | The George Institute |
| NCT04353596 | Stopping ACE-inhibitors in COVID-19 (ACEI-COVID) | COVID-19 | Completed | Phase 4 | Medical University Innsbruck |