Sequence information
DRAVP ID DRAVPc054
Name Compstatin 40(AMY-101)
Sequence
Molecular Formula C83H117N23O18S2
Condition/Disease COVID-19
Group Phase Ⅱ clinical trial
Type Peptide
Description C3 Complement Inhibitor AMY-101 is a compstatin-based inhibitor of human complement component C3, with potential use as a treatment for various diseases in which excessive complement activation plays a key role, including paroxysmal nocturnal hemoglobinuria (PNH) and complement 3 glomerulopathy (C3G). Upon administration, C3 complement inhibitor AMY-101 selectively binds to C3 and inhibits C3 activity. This prevents complement pathway activation, and inhibits complement-mediated inflammation and cell lysis. Excessive complement activation plays a key role in various inflammatory and autoimmune diseases, and leads to tissue destruction. C3 is a crucial and central component of the complement system, and the complement system is an integral component of the innate immune response.
Active sequence/Structure
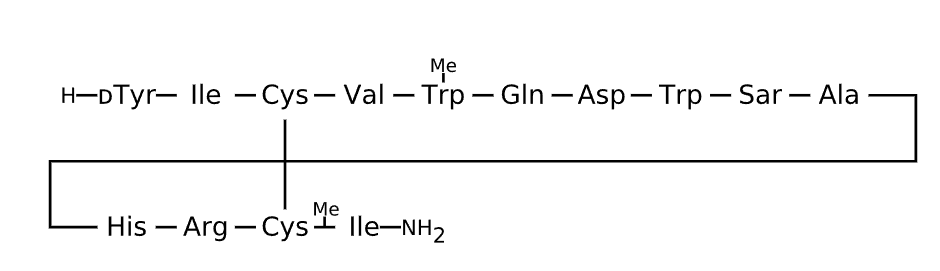
Comment
X is Trp(Me), a methylated form of tryptophan
External Links
DrugBank Accession Number DB14803
Pubchem ID 131634231
CHEMBL ID CHEMBL4297260
UNII 4Z4DFR9BX7
CAS 1427001-89-5
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT04395456 | A Study of the C3 Inhibitor AMY-101 in Patients With ARDS Due to COVID-19 (SAVE) (SAVE) | Acute Respiratory Distress Syndrome Due to SARS-CoV-2 Infection (Severe COVID19) | Unknown | Phase 2 | Amyndas Pharmaceuticals S.A. |
| 2020-001550-22(EU) | A Phase 2 Clinical Trial to Assess the Safety and Efficacy of Complement 3 Inhibitor, AMY-101, in patients with Acute Respiratory Distress Syndrome (ARDS) due to Covid-19. | Acute Respiratory Distress Syndrome (ARDS) due to SARS-CoV-2 infection. | Ongoing | Phase 2 |