Sequence information
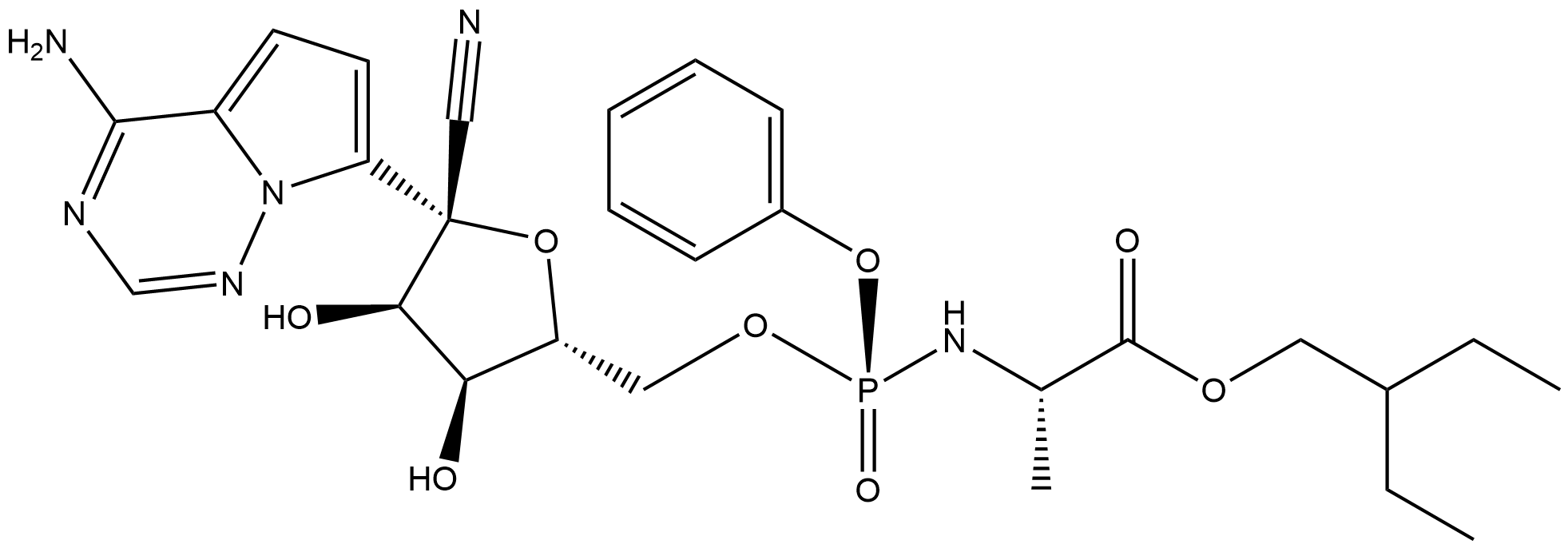
DRAVP ID DRAVPc056
Name Remdesivir
Sequence Not available
Molecular Formula C27H35N6O8P
Condition/Disease COVID-19
Group Approved
Type Peptide like Small molecule
Description Remdesivir (GS-5734) is an adenosine triphosphate analogue first described in the literature in 2016 as a potential treatment for Ebola. Broad antiviral activity of remdesivir is suggested by its mechanism of action, and to date, it has demonstrated in vitro activity against the Arenaviridae, Flaviviridae, Filoviridae, Paramyxoviridae, Pneumoviridae, and Coronaviridae viral families.Remdesivir was confirmed as a non-obligate chain terminator of RdRp from SARS-CoV-2 and the related SARS-CoV and MERS-CoV, and has been investigated in multiple COVID-19 clinical trials.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB14761
Pubchem ID 121304016
CHEMBL ID CHEMBL4065616
UNII 3QKI37EEHE
CAS 1809249-37-3
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT05502081 | Clinical Study to Compare Efficacy and Safety of Casirivimab and Imdevimab Combination, Remdesivir and Favipravir in Hospitalized COVID-19 Patients | COVID-19 | Completed | Phase 4 | Mansoura University Hospital |
| NCT04280705 | Adaptive COVID-19 Treatment Trial (ACTT) | COVID-19 | Completed | Phase 3 | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT04843761 | ACTIV-3b: Therapeutics for Severely Ill Inpatients With COVID-19 (TESICO) | COVID-19 | Completed | Phase 3 | National Institute of Allergy and Infectious Diseases (NIAID) |
| NCT04292899 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) | COVID-19 | Completed | Phase 3 | Gilead Sciences |
| NCT04596839 | Antiviral Activity and Safety of Remdesivir in Bangladeshi Patients With Severe Coronavirus Disease (COVID-19) | COVID-19 | Completed | Phase 3 | Dr. Md. Alimur Reza |