Sequence information
DRAVP ID DRAVPc059
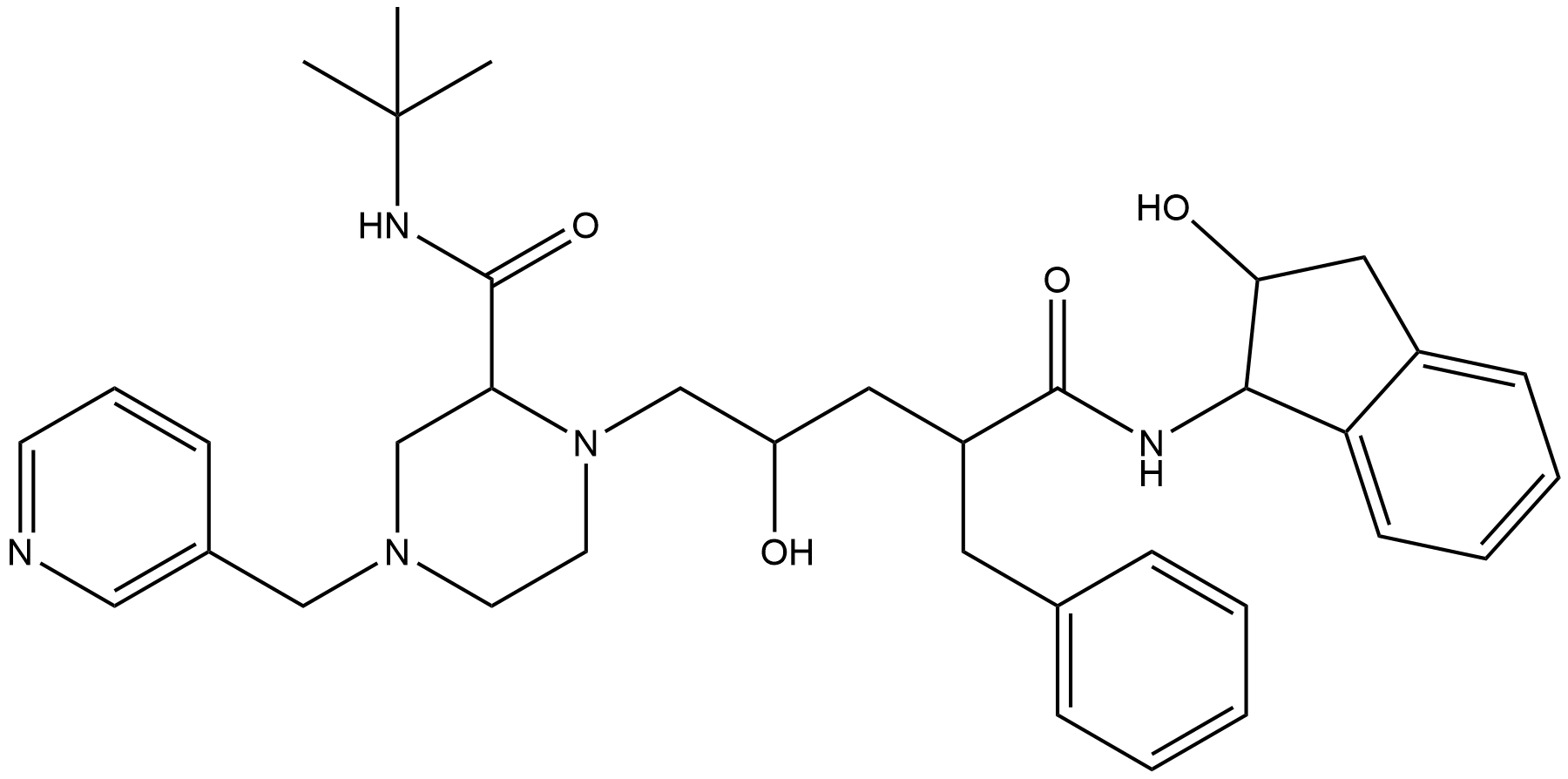
Name Indinavir
Sequence Not available
Molecular Formula C36H47N5O4
Condition/Disease HIV infection.
Group Approved
Type Peptidomimetic
Description Indinavir is a N-(2-hydroxyethyl)piperazine, a piperazinecarboxamide and a dicarboxylic acid diamide. It is an antiretroviral protease inhibitor used in the therapy and prevention of human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). Indinavir can cause transient and usually asymptomatic elevations in serum aminotransferase levels and mild elevations in indirect bilirubin concentration. Indinavir is a rare cause of clinically apparent, acute liver injury. In HBV or HCV coinfected patients, antiretroviral therapy with indinavir may result in an exacerbation of the underlying chronic hepatitis B or C.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB00224
Pubchem ID 5362440
CHEMBL ID CHEMBL115
UNII 9MG78X43ZT
CAS 150378-17-9
Reference 36466430
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02300623 | Intermittent ART in Primary HIV Infection (PHI-IL2) | HIV infections | Completed | Phase 4 | Juan A. Arnaiz |
| NCT00148785 | A Pharmacokinetic (PK) Study of a Combination of Indinavir, Ritonavir, and Amprenavir | HIV infections | Completed | Phase 4 | Emory University |
| NCT00002393 | A Study of Indinavir Taken With or Without DMP 266 | HIV infections | Completed | Phase 3 | Dupont Merck |
| NCT00002208 | A Multicenter, Open-Label, Randomized, 24-Week Study to Compare the Safety and Activity of Indinavir Sulfate, 800 Mg q 8 h Versus 1,200 Mg q 12 h in Combination With Zidovudine and 3TC | HIV infections | Completed | Phase 3 | Merck Sharp & Dohme LLC |