Sequence information
DRAVP ID DRAVPc060
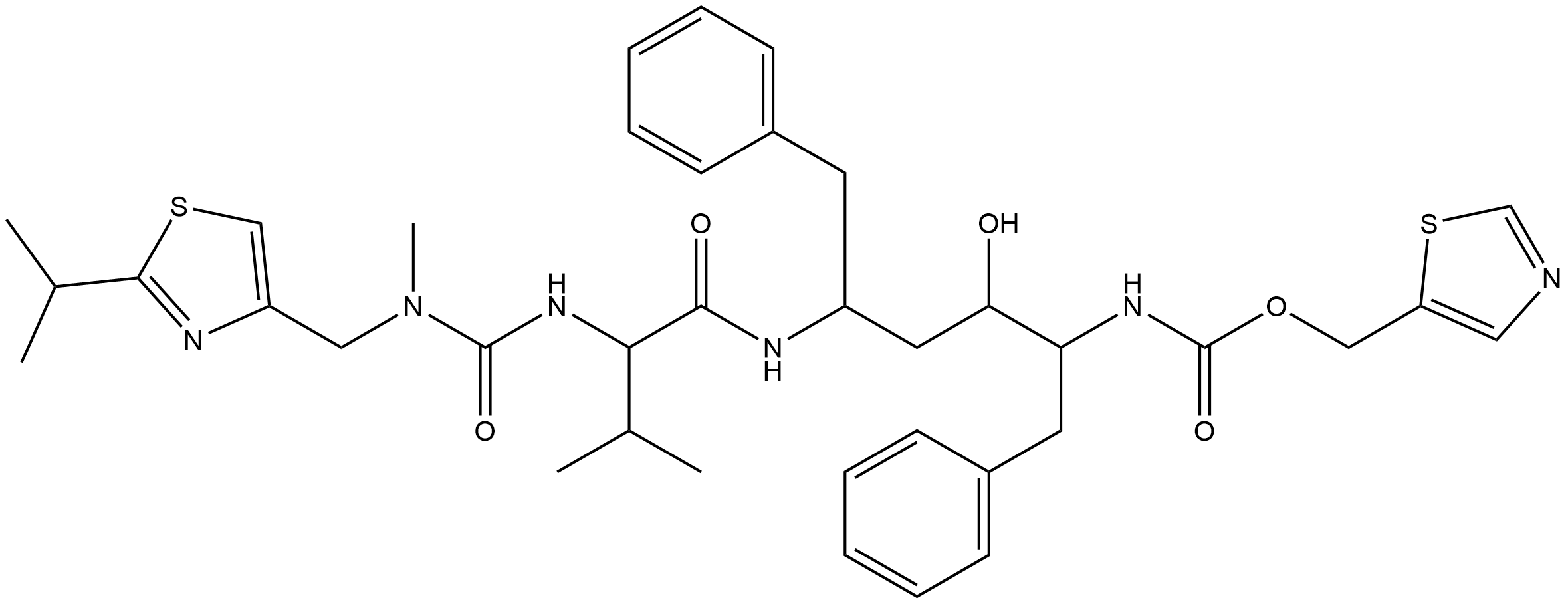
Name Ritonavir
Sequence Not available
Molecular Formula C37H48N6O5S2
Condition/Disease HIV Infection
Group Approved
Type Peptidomimetic
Description Ritonavir is an L-valine derivative that is L-valinamide in which alpha-amino group has been acylated by a [(2-isopropyl-1,3-thiazol-4-yl)methyl]methylcarbamoyl group and in which a hydrogen of the carboxamide amino group has been replaced by a (2R,4S,5S)-4-hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl group. It is an antiretroviral protease inhibitor that is widely used in combination with other protease inhibitors in the therapy and prevention of human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). Ritonavir is used in combination with other drugs to treat coronavirus disease 2019 (COVID-19) in patients who are at risk for progressing into a severe form of the disease.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB00503
Pubchem ID 392622
CHEMBL ID CHEMBL163
UNII O3J8G9O825
CAS 155213-67-5
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00531557 | Double Protease Inhibitor to Darunavir Switch Study | HIV infections | Completed | Phase 4 | St Stephens Aids Trust |
| NCT01232127 | Effects of Famotidine on the Pharmacokinetics of Atazanavir When Coadministered to Participants With HIV Infection | HIV infections | Completed | Phase 4 | Bristol-Myers Squibb |
| NCT02786537 | Study of Oral Treatments for Hepatitis C (PRIORITIZE) | Chronic Hepatitis C | Completed | Phase 4 | University of Florida |