Sequence information
DRAVP ID DRAVPc065
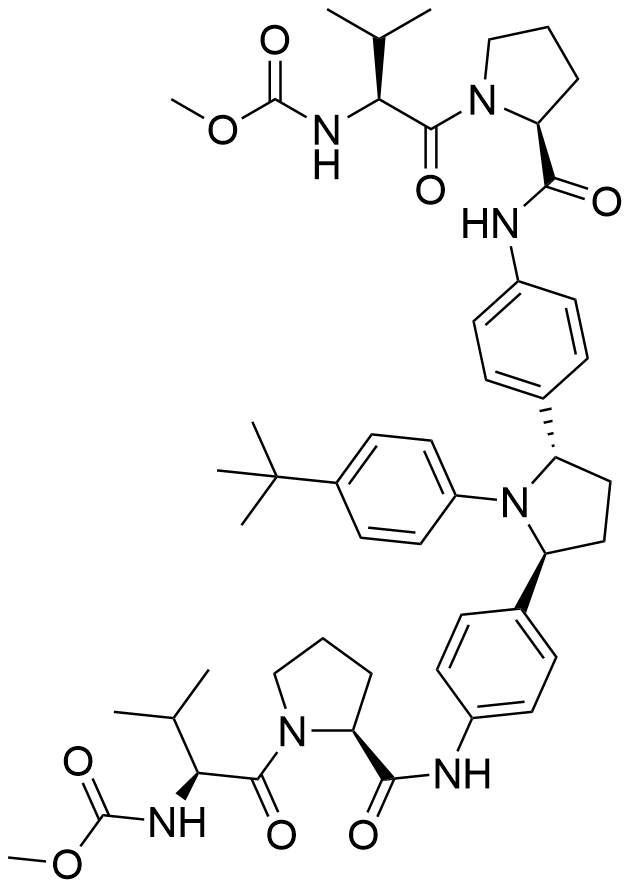
Name Ombitasvir
Sequence Not available
Molecular Formula C50H67N7O8
Condition/Disease Hepatitis C Virus (HCV) infection
Group Approved, Investigational
Type peptide
Description Ombitasvir (ABT-267) is an antiviral drug for the treatment of hepatitis C virus (HCV) infection. Ombitasvir is a potent inhibitor of the hepatitis C virus protein NS5A, has favorable pharmacokinetic characteristics and is active in the picomolar range against genotype 1 - 6. In 2015, it was approved by FDA for use in combination with paritaprevir, ritonavir and dasabuvir in the product Viekira Pak for the treatment of HCV genotype 1.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB09296
Pubchem ID 54767916
CHEMBL ID CHEMBL3127326
UNII EQE3I70J3W
CAS 1258226-87-7
Reference 27179128 24400777 27401997 28497432 15302943 25585348 9305675
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02886624 | Short Duration Therapy of Acute Hepatitis C Genotypes 1 or 4 (SAHIV) | Acute Hepatitis C; HIV | Completed | Phase 2 | Institut de Médecine et d'Epidémiologie Appliquée - Fondation Internationale Léon M'Ba |
| NCT02940691 | Scale-up of Treatment of Hepatitis C Infection Among People Who Inject Drugs (DARLO-C) | Hepatitis C | Terminated | Phase 4 | Kirby Institute |
| NCT04378608 | Ombitasvir /Paritaprevir/Ritonavir Plus Ribavirin on HCV GT4 | Hepatitis C Virus Infection | Completed | Phase 1,2 | Beni-Suef University |