Sequence information
DRAVP ID DRAVPc066
Name Lopinavir
Sequence Not available
Molecular Formula C37H48N4O5
Condition/Disease human immunodeficiency virus (HIV) infection.
Group Approved
Type peptidomimetic
Description Lopinavir is an antiretroviral protease inhibitor used in combination with other antiretrovirals in the treatment of HIV-1 infection. Lopinavir is marketed and administered exclusively in combination with ritonavir - this combination, first marketed by Abbott under the brand name Kaletra in 2000, is necessary due to lopinavir's poor oral bioavailability and extensive biotransformation. Ritonavir is a potent inhibitor of the enzymes responsible for lopinavir metabolism, and its co-administration "boosts" lopinavir exposure and improves antiviral activity.Like many other protease inhibitors (e.g. saquinavir, nelfinavir), lopinavir is a peptidomimetic molecule - it contains a hydroxyethylene scaffold that mimics the peptide linkage typically targeted by the HIV-1 protease enzyme but which itself cannot be cleaved, thus preventing the activity of the HIV-1 protease.
Active sequence/Structure
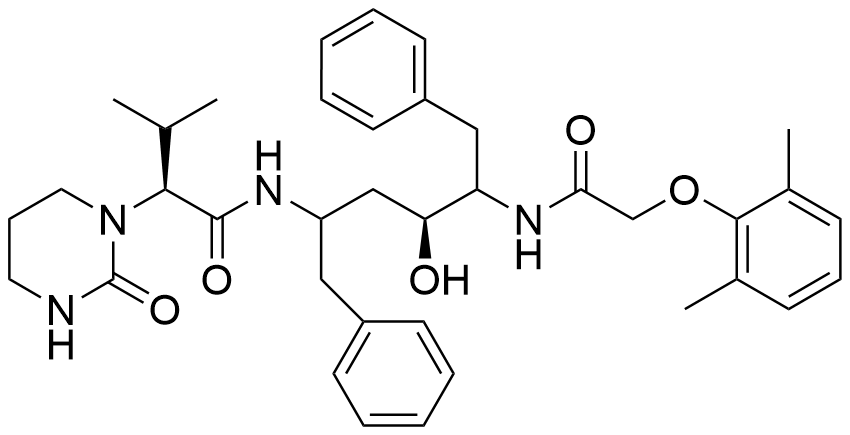
Comment
No comments found.
External Links
DrugBank Accession Number DB01601
Pubchem ID 92727
CHEMBL ID CHEMBL729
UNII 2494G1JF75
CAS 192725-17-0
Reference 9884314 19108994 9835517 30346663 22762019 21953914
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT01314261 | Study of ABT-267 in Treatment Naive Hepatitis C Virus (HCV) Genotype 1 Infected Subjects | Chronic Hepatitis C,Hepatitis C Virus (HCV) Infection | Completed | Phase 2 | AbbVie (prior sponsor, Abbott) |
| NCT02247401 | Coadministration of ABT-450/Ritonavir/ABT-267 (ABT-450/r/ABT-267) With Ribavirin (RBV) in Adults With Genotype 4 (GT4) Hepatitis C Virus (HCV) in Egypt | HCV;Hepatitis C Infection;Genotype 4 | Completed | Phase 3 | AbbVie |
| NCT01159275 | Lopinavir (LPV) Dose Reduction | HIV-1 Infections | Completed | Phase 1, Phase 2 | The HIV Netherlands Australia Thailand Research Collaboration |