Sequence information
DRAVP ID DRAVPc068
Name Grazoprevir
Sequence Not available
Molecular Formula C38H50N6O9S
Condition/Disease hepatitis C virus infections.
Group Approved
Type Cyclic peptidomimetic
Description Grazoprevir is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV).In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Grazoprevir as first line therapy in combination with Elbasvir for genotypes 1a, 1b, and 4 of Hepatitis C.
Active sequence/Structure
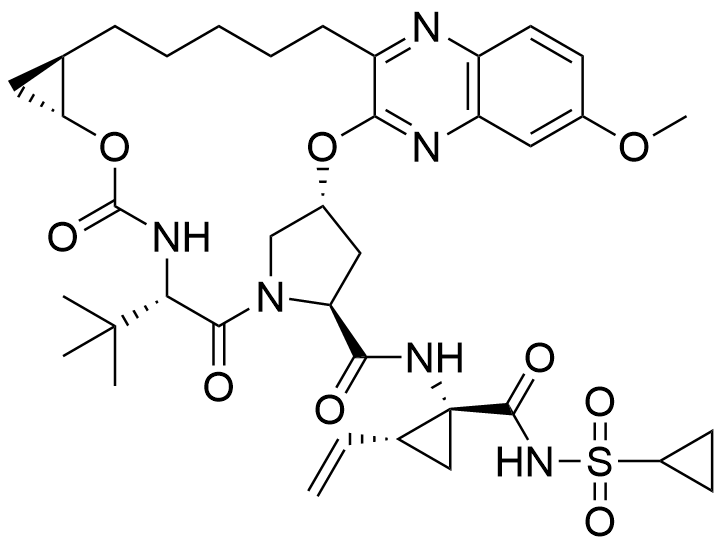
Comment
No comments found.
External Links
DrugBank Accession Number DB11575
Pubchem ID 44603531
CHEMBL ID CHEMBL2063090
UNII 8YE81R1X1J
CAS 1350514-68-9
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT06172660 | Real-World Effectiveness of Perinatal RSV Immunoprophylaxis | RSV Infection | Recruiting | Yale University | |
| NCT04840849 | Evaluate the Pharmacokinetics, Safety, and Tolerability of Nirsevimab in Healthy Chinese Adults (PK/ADA) | Evaluate PK Profile | Completed | Phase 1 | AstraZeneca |
| NCT03420300 | EBR/GZR for HCV-1b Patients Receiving Hemodialysis | Hepatitis C | Completed | Phase 4 | National Taiwan University Hospital |