Sequence information
DRAVP ID DRAVPc069
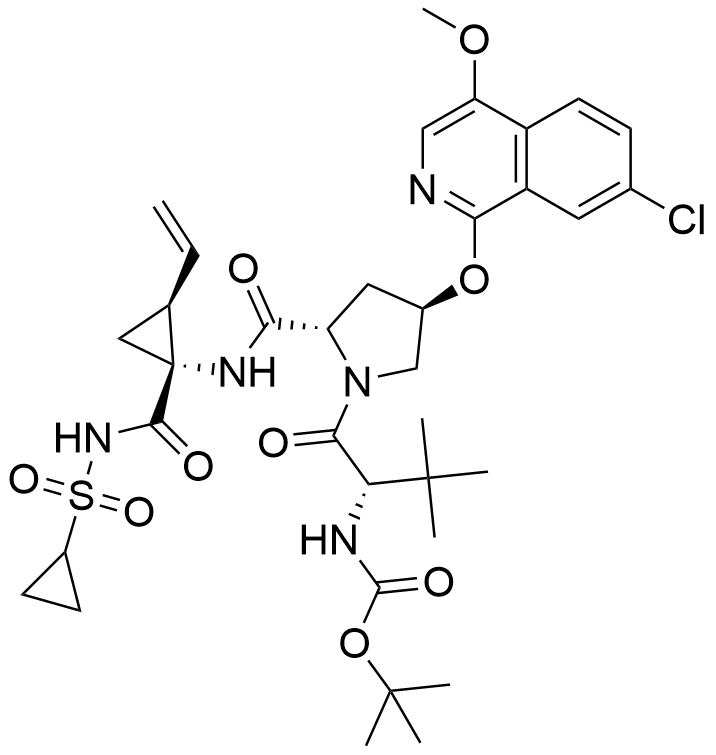
Name Asunaprevir
Sequence Not available
Molecular Formula C35H46ClN5O9S
Condition/Disease hepatitis C genotype 1b
Group Approved, Investigational, Withdrawn
Type peptide
Description Asunaprevir, also named BMS-650032, is a potent hepatitis C virus (HCV) NS3 protease inhibitor. It has been shown to have a very high efficacy in dual-combination regimens with daclatasvir in patients chronically infected with HCV genotype 1b. It was developed by Bristol-Myers Squibb Canada and approved by Health Canada on April 22, 2016. The commercialization of asunaprevir was cancelled one year later on October 16, 2017.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB11586
Pubchem ID 16076883
CHEMBL ID CHEMBL2105735
UNII S9X0KRJ00S
CAS 630420-16-5
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT00940771 | Equivalence of Boosted Atazanavir Based Regimens and Currently Effective HAART Regimens | Pediatric HIV; HIV Infections | Completed | Phase 4 | Phoenix Children's Hospital |
| NCT01511809 | Efficacy of Atazanavir/Ritonavir Monotherapy as Maintenance in Patients With Viral Suppression | HIV-1 Infection | Completed | Phase 3 | IRCCS San Raffaele |
| NCT00307502 | Study to Determine the Pharmacokinetic Behavior of Antiretroviral Drugs in Patients Infected by HIV | HIV Infections | Completed | Phase 1 | Germans Trias i Pujol Hospital |