Sequence information
DRAVP ID DRAVPc070
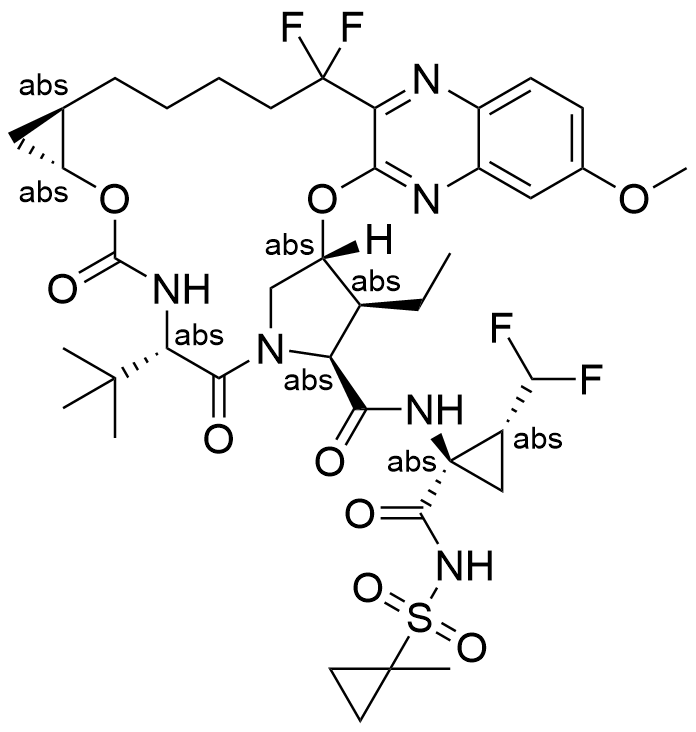
Name Voxilaprevir
Sequence Not available
Molecular Formula C40H52F4N6O9S
Condition/Disease Hepatitis C infections
Group Approved, Investigational
Type Cyclic peptidomimetic
Description Voxilaprevir is a Direct-Acting Antiviral (DAA) medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV).Voxilaprevir has been available since July 2017 in a fixed dose combination product with sofosbuvir and velpatasvir as the commercially available product Vosevi. Vosevi is approved for the treatment of adult patients with chronic HCV infection with genotype 1, 2, 3, 4, 5, or 6 infection.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB12026
Pubchem ID 89921642
CHEMBL ID CHEMBL4474855
UNII 0570F37359
CAS 1535212-07-7
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02402452 | Pharmacokinetics of Voxilaprevir in Adults With Normal Renal Function and Severe Renal Impairment | HCV Infection | Completed | Phase 1 | Gilead Sciences |
| NCT02745535 | Safety, Tolerability and Efficacy of Sofosbuvir, Velpatasvir, and Voxilaprevir in Subjects With Previous DAA Experience (RESOLVE) | Chronic Hepatitis C | Completed | Phase 2 | University of Maryland, Baltimore |
| NCT03888729 | Simplifying HCV Treatment in Rwanda for Elsewhere in the Developing World: Pangenotypic and Retreatment Study (SHARED3) (SHARED3) | Hepatitis C, Chronic | Unknown status | Phase 4 | Partners in Health |