Sequence information
DRAVP ID DRAVPc073
Name nirmatrelvir
Sequence Not available
Molecular Formula C23H32F3N5O4
Condition/Disease COVID-19
Group Approved, Investigational
Type peptidomimetic
Description Nirmatrelvir (PF-07321332) is an orally bioavailable 3C-like protease (3CLPRO) inhibitor that is the subject of clinical trial NCT04756531.In December 2021, the FDA granted an emergency use authorization to Paxlovid, a co-packaged product containing both nirmatrelvir and ritonavir, for the treatment of certain patients with mild-to-moderate COVID-19.Paxlovid was approved for use in Canada in January 2022 for the treatment of adult patients with mild-moderate COVID-19 and later granted conditional marketing authorization by the European Commission on January 27, 2022.
Active sequence/Structure
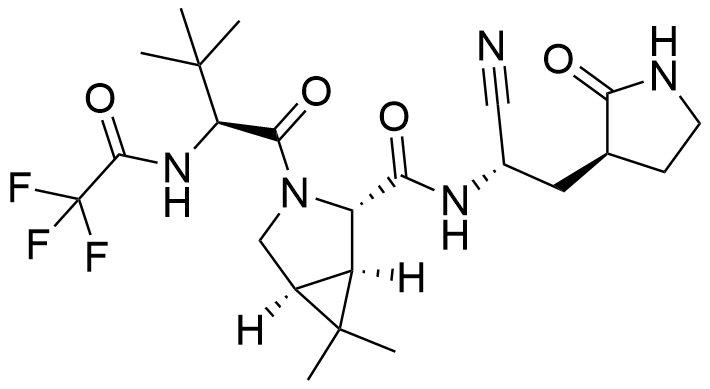
Comment
No comments found.
External Links
DrugBank Accession Number DB16691
Pubchem ID 155903259
CHEMBL ID CHEMBL4802135
UNII 7R9A5P7H32
CAS 2628280-40-8
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT05813600 | Efficacy and Safety of Nirmatrelvir/Ritonavir for Treating Omicron Variant of COVID-19 | Omicron Variant of COVID-19 | Completed | Not Applicable | Xiangao Jiang |
| NCT05567952 | A Study to Learn About a Repeat 5-Day Treatment With the Study Medicines (Called Nirmatrelvir/Ritonavir) in People 12 Years Old or Older With Return of COVID-19 Symptoms and SARS-CoV-2 Positivity Aft | COVID-19 | Completed | Phase 2 | Pfizer |
| NCT05261139 | EPIC-Peds: A Study to Learn About the Study Medicine Called PF-07321332 (Nirmatrelvir)/Ritonavir in Patients Under 18 Years of Age With COVID-19 That Are Not Hospitalized But Are at Risk for Severe D | COVID-19 | Recruiting | Phase 2,Phase 3 | Pfizer |
| NCT05386472 | A Study to Learn About the Study Medicine (Nirmatrelvir Plus Ritonavir) in Pregnant Women With COVID-19 | COVID-19 | Recruiting | Phase 1 | Pfizer |
| NCT05668091 | A Decentralized, Randomized Phase 2 Efficacy and Safety Study of Nirmatrelvir/Ritonavir in Adults with Long COVID. | Long COVID | Completed | Phase 2 | Harlan M Krumholz |
| NCT05852873 | PAxlovid loNg cOvid-19 pRevention triAl With recruitMent In the Community in Norway (PanoramicNOR) | Post COVID-19 Condition, Unspecified,SARS-CoV2 Infection,COVID-19 | Recruiting | Phase 3 | Haukeland University Hospital |