Sequence information
DRAVP ID DRAVPc074
Name simeprevir
Sequence Not available
Molecular Formula C38H47N5O7S2
Condition/Disease HCV
Group Approved
Type Cyclic peptidomimetic
Description Simeprevir is a hepatitis C virus (HCV) NS3/4A protease inhibitor indicated in patient's with HCV genotype 1 for the treatment of chronic hepatitis C virus (HCV) infection. Simeprevir was approved by the FDA in November 2014 and is marketed under the brand name Olysio as oral tablets. Administered once daily with food, 150mg simeprevir capsule is used in combination with Sofosbuvir in patients with HCV genotype 1 without cirrhosis .
Active sequence/Structure
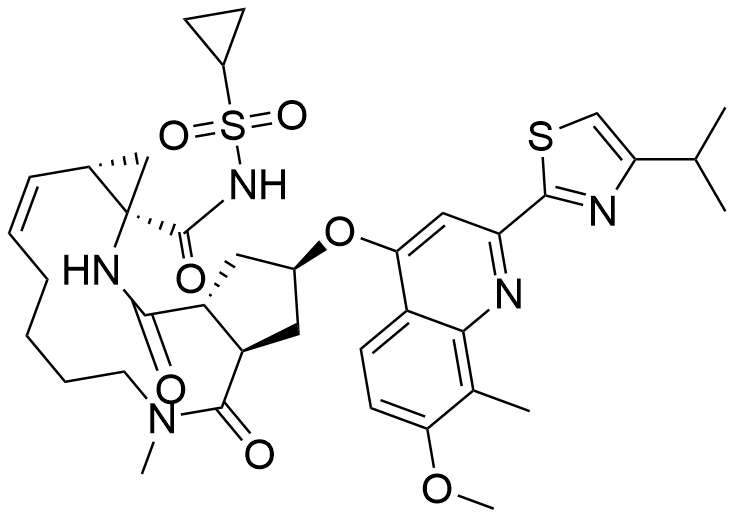
Comment
No comments found.
External Links
DrugBank Accession Number DB06290
Pubchem ID 24873435
CHEMBL ID CHEMBL501849
UNII 9WS5RD66HZ
CAS 923604-59-5
Reference 25585348 20166108 26694454 25206310 24920913 18678486
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02404805 | Drug Interaction Potential Between Dolutegravir and Simeprevir in HIV/HCV Seronegative Volunteers | HIV,Hepatitis C | Completed | Not Applicable | University of Colorado, Denver |
| NCT01241773 | TMC435-TiDP16-C123 - A Study in Healthy Volunteers Investigating the Pharmacokinetic Interaction Between TMC435 and the Antiretroviral Agents Efavirenz and Raltegravir | Hepatitis C Virus | Completed | Phase 1 | Tibotec Pharmaceuticals, Ireland |
| NCT02114177 | Efficacy and Safety Study of Simeprevir in Combination With Sofosbuvir in Participants With Chronic Hepatitis C Virus Infection Without Cirrhosis | Hepatitis C Virus Infection | Completed | Phase 3 | Janssen Infectious Diseases BVBA |
| NCT00752648 | TMC435350-TiDP16-C106: A Phase I Trial to Compare the Bioavailability and Plasma Pharmacokinetics After a Single Oral Dose of TMC435350 of 2 Different Solid Formulations Relative to a Powder Blend Cap | Hepatitis C,HCV | Completed | Phase 1 | Tibotec Pharmaceuticals, Ireland |
| NCT02268864 | A Study to Assess the Efficacy and Safety of the Combination of Simeprevir and Daclatasvir in Chronic Hepatitis C Genotype 1b-Infected Participants (COMMIT) | Hepatitis C, Chronic | Completed | Phase 2 | Janssen-Cilag International NV |