Sequence information
DRAVP ID DRAVPc075
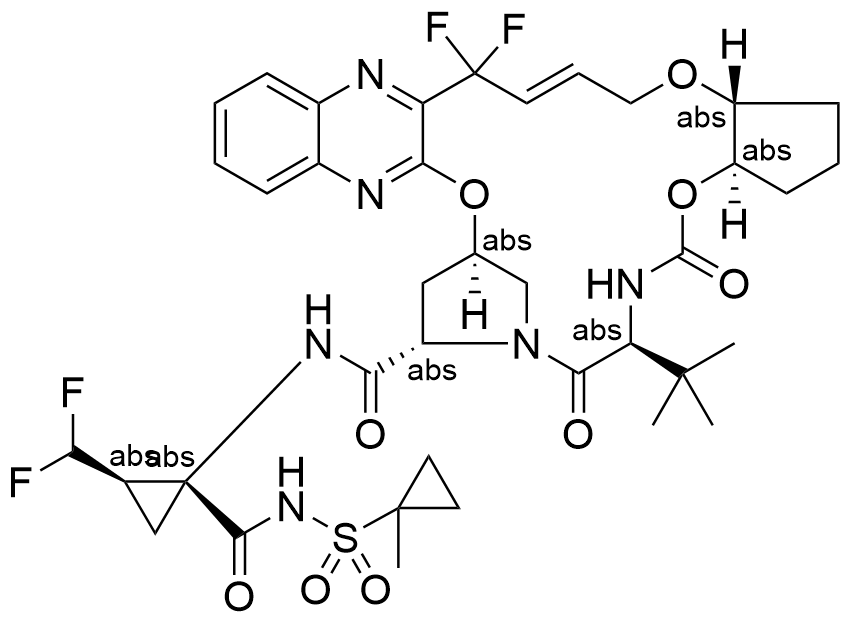
Name glecaprevir
Sequence Not available
Molecular Formula C38H46F4N6O9S
Condition/Disease HCV
Group Approved, Investigational
Type Cyclic peptidomimetic
Description Glecaprevir is a direct acting antiviral agent and Hepatitis C virus (HCV) NS3/4A protease inhibitor that targets the the viral RNA replication. Glecaprevir is available as an oral combination therapy with Pibrentasvir under the brand name Mavyret. This fixed-dose combination therapy was FDA-approved in August 2017.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB13879
Pubchem ID 66828839
CHEMBL ID CHEMBL3545363
UNII K6BUU8J72P
CAS 1365970-03-1
Reference 24282816
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02692703 | A Study to Evaluate the Safety and Efficacy of ABT-493/ABT-530 in Adult Post-Liver or Post-Renal Transplant Recipients With Chronic Hepatitis C Virus (MAGELLAN-2) | Chronic Hepatitis C, HCV, Hepatitis C Virus | Completed | Phase 3 | AbbVie |
| NCT02966795 | A Study of of Glecaprevir/Pibrentasvir in Adults With Chronic Hepatitis C Virus (HCV) Genotype 5 or 6 Infection (ENDURANCE-5 6) | Hepatitis C Virus (HCV) | Completed | Phase 3 | AbbVie |
| NCT03235349 | Efficacy and Safety of Glecaprevir/Pibrentasvir (ABT-493/ABT-530) in Treatment-Naive and Treatment-Experienced Asian Adults With Chronic Hepatitis C Virus Genotype (GT) 1 to GT6 Infection With Compe | Hepatitis C Virus (HCV) | Completed | Phase 3 | AbbVie |
| NCT02243293 | A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Co-Administration of ABT-493 and ABT-530 With and Without RBV in Subjects With Chronic Hepatitis C Virus (HCV) Genotypes 2, 3, 4, 5 or | Chronic Hepatitis C, Hepatitis C Virus | Completed | Phase 2,Phase 3 | AbbVie |