Sequence information
DRAVP ID DRAVPc076
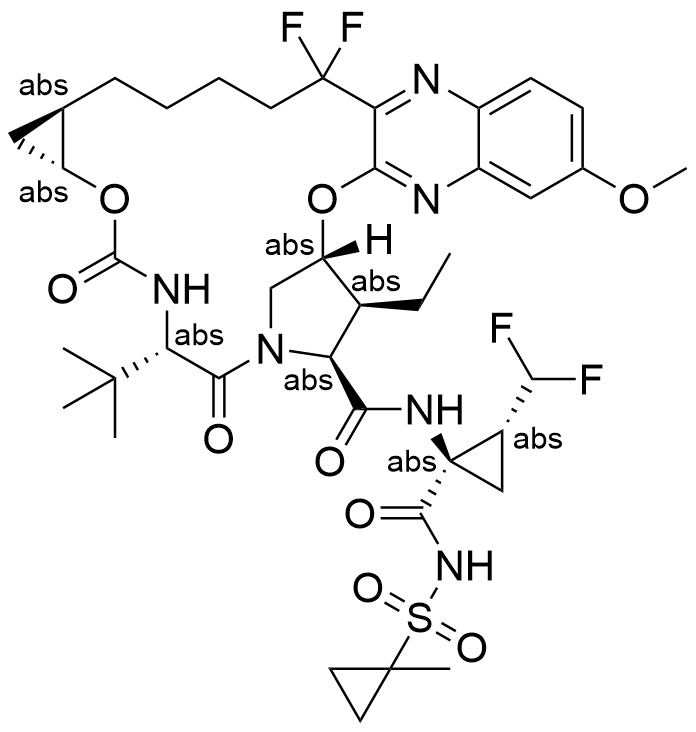
Name voxilaprevir
Sequence Not available
Molecular Formula C40H52F4N6O9S
Condition/Disease HCV
Group Approved, Investigational
Type Cyclic peptidomimetic
Description Voxilaprevir is a Direct-Acting Antiviral (DAA) medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). Voxilaprevir has been available since July 2017 in a fixed dose combination product with sofosbuvir and velpatasvir as the commercially available product Vosevi.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB12026
Pubchem ID 89921642
CHEMBL ID CHEMBL4474855
UNII 0570F37359
CAS 1535212-07-7
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT02402452 | Pharmacokinetics of Voxilaprevir in Adults With Normal Renal Function and Severe Renal Impairment | HCV Infection | Completed | Phase 1 | Gilead Sciences |
| NCT02185794 | Study to Evaluate Safety, Tolerability, Pharmacokinetics, and Antiviral Activity of Voxilaprevir in Adults With Chronic Hepatitis C Virus Infection | Hepatitis C Virus Infection | Completed | Phase 1 | Gilead Sciences |
| NCT02745535 | Safety, Tolerability and Efficacy of Sofosbuvir, Velpatasvir, and Voxilaprevir in Subjects With Previous DAA Experience (RESOLVE) | Chronic Hepatitis C | Completed | Phase 2 | University of Maryland, Baltimore |
| NCT04695769 | Combined Ribavirin With Sofosbuvir/Velpatasvir/Voxilaprevir in Retreatment of Chronic Hepatitis C Non-responders | Chronic Hepatitis C | Completed | Phase 4 | Helwan University |
| NCT02378961 | Safety and Efficacy of Voxilaprevir Plus Sofosbuvir/Velpatasvir Fixed Dose Combination in Adults With Chronic Non-Genotype 1 HCV Infection | Hepatitis C Virus Infection | Completed | Phase 2 | Gilead Sciences |
| NCT04211909 | Study to Investigate the Efficacy and Safety of Sofosbuvir/Velpatasvir (SOF/VEL) Fixed-Dose Combination (FDC) and Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX ) FDC for 12 Weeks in Adults Wi | Hepatitis C Virus Infection | Completed | Phase 3 | Gilead Sciences |