Sequence information
DRAVP ID DRAVPc077
Name daclatasvir
Sequence Not available
Molecular Formula C40H50N8O6
Condition/Disease HCV
Group Approved, Investigational
Type peptidomimetic
Description Daclatasvir is a direct-acting antiviral agent against Hepatitis C Virus (HCV) used for the treatment of chronic HCV genotype 1 and 3 infection.According to 2017 American Association for the Study of Liver Diseases (AASLD), 60mg of daclatasvir is recommended with 400mg Sofosbuvir for genotype 1a/b patients with or without cirrhosis as second-line therapy.
Active sequence/Structure
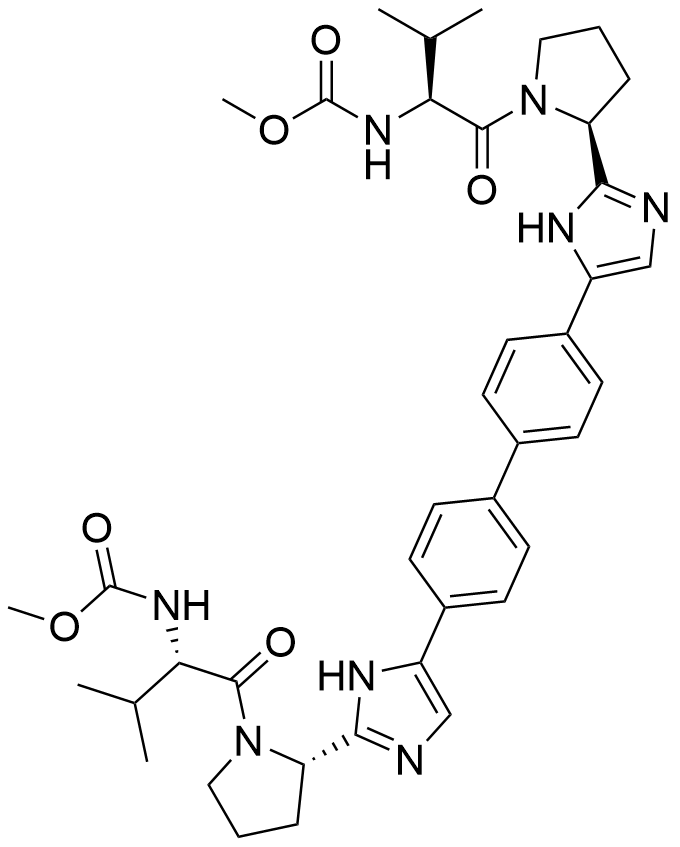
Comment
No comments found.
External Links
DrugBank Accession Number DB09102
Pubchem ID 25154714
CHEMBL ID CHEMBL2023898
UNII LI2427F9CI
CAS 1009119-64-5
Reference 27029743 25585348 25046163 24204123 26486762 23431163 24521299
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT01830205 | Pharmacokinetic and Safety Study of Daclatasvir in Patients With Renal Impairment | Hepatitis C | Completed | Phase 1 | Bristol-Myers Squibb |
| NCT00874770 | Safety and Efficacy of Daclatasvir (BMS-790052) Plus Standard of Care (Pegylated-interferon Alpha and Ribavirin) | Hepatitis C Infection | Completed | Phase 2 | Bristol-Myers Squibb |
| NCT02323594 | A Bioequivalence Study of Daclatasvir Tablets and Bioavailability Studies of Daclatasvir and Asunaprevir | Hepatitis C Infection | Completed | Phase 1 | Bristol-Myers Squibb |
| NCT00859053 | Single-Dose Pharmacokinetics of BMS-790052 in Participants With Hepatic Impairment | Hepatic Insufficiency | Completed | Phase 1 | Bristol-Myers Squibb |