Sequence information
DRAVP ID DRAVPc078
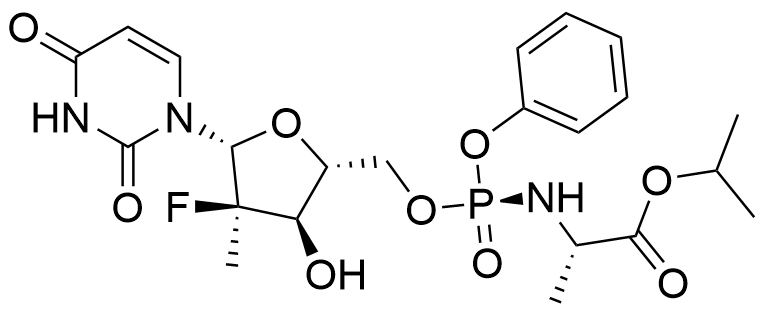
Name sofosbuvir
Sequence Not available
Molecular Formula C22H29FN3O9P
Condition/Disease HCV
Group Approved
Type peptidomimetic
Description Sofosbuvir (tradename Sovaldi) is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV).Since 2014, sofosbuvir has been available as a fixed dose combination product with Ledipasvir (tradename Harvoni) used for the treatment of chronic Hepatitis C. Approved in October 2014 by the FDA, Harvoni is indicated for the treatment of HCV genotypes 1, 4, 5, and 6 with or without Ribavirin depending on the level of liver damage or cirrhosis Label.
Active sequence/Structure
Comment
No comments found.
External Links
DrugBank Accession Number DB08934
Pubchem ID 45375808
CHEMBL ID CHEMBL1259059
UNII WJ6CA3ZU8B
CAS 1190307-88-0
Reference 26426038 27482432 9305675 28248189 23262999 26196665 25585348 28497432 15483230 25659285 24733478 24289735 20801890
ClinicalTrails Information
| NCT Number | Study Title | Condition/Disease | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT04460443 | Sofosbuvir in Treatment of COVID 19 | COVID | Unknown status | Phase 2,Phase 3 | Tanta University |
| NCT03282474 | HepNet Pilot Trial: Multicenter Trial for the Treatment of Chronic Hepatitis E With Sofosbuvir (SofE) | Hepatitis E,Hepatitis Chronic Viral | Completed | Phase 2 | Hannover Medical School |
| NCT03312023 | Ledipasvir/Sofosbuvir for Hepatitis B Virus Infection (APOSTLE) | Hepatitis B | Completed | Phase 2 | University of Maryland, Baltimore |
| NCT02836925 | Ledipasvir+Sofosbuvir and Sofosbuvir+Velpatasvir for Pts With Indolent Bcell Lymphoma Associated With HCV Infection | Indolent B-cell Lymphoma, Hepatitis C | Completed | Phase 2 | Fondazione Italiana Linfomi - ETS |